Abstract
The herpes simplex virus type 1 (HSV-1) VP22, is one of the most abundant HSV-1 tegument proteins with an average stoichiometry of 2 400 copies per virion and conserved among alphaherpesvirinae. Many functions are attributed to VP22, including nuclear localization, chromatin binding, microtubule binding, induction of microtubule reorganization, intercellular transport, interaction with cellular proteins, such as template activating factor I (TAF-I) and nonmuscle myosin II A (NMIIA), and viral proteins including tegument protein VP16, pUS9 and pUL46, glycoprotein E (gE) and gD. Recently, many novel functions performed by the HSV-1 VP22 protein have been shown, including promotion of protein synthesis at late times in infection, accumulation of a subset of viral mRNAs at early times in infection and possible transcriptional regulation function.
Similar content being viewed by others
References
Aints A, Guven H, Gahrton G, et al. 2001. Mapping of herpes simplex virus-1 vp22 functional domains for inter- and subcellular protein targeting. Gene Ther, 8(14): 1051–1056.
Beerens A M, Rots M G, de Vries E F, et al. 2007. Fusion of herpes simplex virus thymidine kinase to vp22 does not result in intercellular trafficking of the protein. Int J Mol Med, 19(5): 841–849.
Bian J, Kiedrowski M, Mal N, et al. 2006. Engineered cell therapy for sustained local myocardial delivery of nonsecreted proteins. Cell Transplant, 15(1): 67–74.
Bian J, Popovic Z B, Benejam C, et al. 2007. Effect of cell-based intercellular delivery of transcription factor gata4 on ischemic cardiomyopathy. Circ Res, 100(11): 1626–1633.
Brandimarti R, Roizman B. 1997. Us9, a stable lysineless herpes simplex virus 1 protein, is ubiquitinated before packaging into virions and associates with proteasomes. Proc Natl Acad Sci USA, 94(25): 13973–13978.
Brignati M J, Loomis J S, Wills J W, et al. 2003. Membrane association of vp 22, a herpes simplex virus type 1 tegument protein. J Virol, 77(8): 4888–4898.
Duffy C, Mbong E F, Baines J D. 2009. Vp22 of herpes simplex virus 1 promotes protein synthesis at late times in infection and accumulation of a subset of viral mrnas at early times in infection. J Virol, 83(2): 1009–1017.
Duffy C, Lavail J H, Tauscher A N, et al. 2006. Characterization of a ul49-null mutant: Vp22 of herpes simplex virus type 1 facilitates viral spread in cultured cells and the mouse cornea. J Virol, 80(17): 8664–8675.
Elliott G, O’Hare P. 1997. Intercellular trafficking and protein delivery by a herpesvirus structural protein. Cell, 88(2): 223–233.
Elliott G, P. Hare O’. 1998. Herpes simplex virus type 1 tegument protein vp22 induces the stabilization and hyperacetylation of microtubules. J Virol, 72(8): 6448–6455.
Elliott G, O’Hare P. 1999. Intercellular trafficking of vp22-gfp fusion proteins. Gene Ther, 6(1): 149–151.
Elliott G, O’Hare P. 1999. Live-cell analysis of a green fluorescent protein-tagged herpes simplex virus infection. J Virol, 73(5): 4110–4119.
Elliott G, Mouzakitis G, O’Hare P. 1995. Vp16 interacts via its activation domain with vp22, a tegument protein of herpes simplex virus, and is relocated to a novel macromo-lecular assembly in coexpressing cells. J Virol, 69(12): 7932–7941.
Elliott G, O’Reilly D, O’Hare P. 1999. Identification of phosphorylation sites within the herpes simplex virus tegument protein vp22. J Virol 73(7): 6203–6206.
Elliott G, Hafezi W, Whiteley A, et al. 2005. Deletion of the herpes simplex virus vp22-encoding gene (ul49) alters the expression, localization, and virion incorporation of icp0. J Virol, 79(15): 9735–9745.
Elliott G D, Meredith D M. 1992. The herpes simplex virus type 1 tegument protein vp22 is encoded by gene ul49. J Gen Virol, 73(Pt 3): 723–726.
Harms J S, Ren X, Oliveira S C, et al. 2000. Distinctions between bovine herpesvirus 1 and herpes simplex virus type 1 vp22 tegument protein subcellular associations. J Virol, 74(7): 3301–3312.
Heine J W, Honess R W, Cassai E, et al. 1974. Proteins specified by herpes simplex virus. Xii. The virion poly-peptides of type 1 strains. J Virol, 14(3): 640–651.
Hutchinson I, Whiteley A, Browne H, et al. 2002. Sequential localization of two herpes simplex virus tegument proteins to punctate nuclear dots adjacent to icp0 domains. J Virol, 76(20): 10365–10373.
Kim T W, Hung C F, Kim J W, et al. 2004. Vaccination with a DNA vaccine encoding herpes simplex virus type 1 vp22 linked to antigen generates long-term antigen-specific cd8-positive memory t cells and protective immunity. Hum Gene Ther, 15(2): 167–177.
Kotsakis A, Pomeranz L E, Blouin A, et al. 2001. Microtubule reorganization during herpes simplex virus type 1 infection facilitates the nuclear localization of vp22, a major virion tegument protein. J Virol, 75(18): 8697–8711.
LaVail J H, Tauscher A N, Sucher A, et al. 2007. Viral regulation of the long distance axonal transport of herpes simplex virus nucleocapsid. Neuroscience, 146(3): 974–985.
Lee J H, Vittone V, Diefenbach E, et al. 2008. Identification of structural protein-protein interactions of herpes simplex virus type 1. Virology, 378(2): 347–354.
Lemken M L, Graepler F, Wolf C, et al. 2007. Fusion of hsv-1 vp22 to a bifunctional chimeric supercd suicide gene compensates for low suicide gene transduction efficiencies. Int J Oncol, 30(5): 1153–1161.
Lemken M L, Wolf C, Wybranietz W A, et al. 2007. Evidence for intercellular trafficking of vp22 in living cells. Mol Ther, 15(2): 310–319.
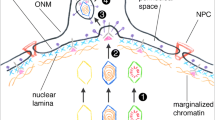
Lopez M R, Schlegel E F, Wintersteller S, et al. 2008. The major tegument structural protein vp22 targets areas of dispersed nucleolin and marginalized chromatin during productive herpes simplex virus 1 infection. Virus Res, 136(1–2): 175–188.
Lundberg M, Johansson M. 2001. Is vp22 nuclear homing an artifact? Nat Biotechnol, 19(8): 713–714.
Martin A, O’Hare P, McLauchlan J, et al. 2002. Herpes simplex virus tegument protein vp22 contains overlapping domains for cytoplasmic localization, microtubule interaction, and chromatin binding. J Virol, 76(10): 4961–4970.
Mettenleiter T C. 2002. Herpesvirus assembly and egress. J Virol, 76(4): 1537–1547.
Miranda-Saksena M, Boadle R A, Armati P, et al. 2002. In rat dorsal root ganglion neurons, herpes simplex virus type 1 tegument forms in the cytoplasm of the cell body. J Virol, 76(19): 9934–9951.
Miyaji-Yamaguchi M, Okuwaki M, Nagata K. 1999. Coiled-coil structure-mediated dimerization of template activating factor-i is critical for its chromatin remodeling activity. J Mol Biol, 290(2): 547–557.
Mori T, Mineta Y, Aoyama Y, et al. 2008. Efficient secretion of the herpes simplex virus tegument protein vp22 from living mammalian cells. Arch Virol, 153(6): 1191–1195.
Mouzakitis G, McLauchlan J, Barreca C, et al. 2005. Characterization of vp22 in herpes simplex virus-infected cells. J Virol, 79(19): 12185–12198.
Murphy M A, Bucks M A, O’Regan K J, et al. 2008. The hsv-1 tegument protein pul46 associates with cellular membranes and viral capsids. Virology, 376(2): 279–289.
Perkins S D, Hartley M G, Lukaszewski R A, et al. 2005. Vp22 enhances antibody responses from DNA vaccines but not by intercellular spread. Vaccine, 23(16): 1931–1940.
Pomeranz L E, Blaho J A. 1999. Modified vp22 localizes to the cell nucleus during synchronized herpes simplex virus type 1 infection. J Virol, 73(8): 6769–6781.
Pomeranz L E, Blaho J A. 2000. Assembly of infectious herpes simplex virus type 1 virions in the absence of full-length vp22. J Virol, 74(21): 10041–10054.
Posnett D N, Engelhorn M E, Lin Y, et al. 2009. Development of effective vaccines for old mice in a tumor model. Vaccine, 27(7): 1093–1100.
Potel C, Elliott G. 2005. Phosphorylation of the herpes simplex virus tegument protein vp22 has no effect on incorporation of vp22 into the virus but is involved in optimal expression and virion packaging of icp0. J Virol, 79(22): 14057–14068.
Rutjes S A, Bosma P J, Rohn J L, et al. 2003. Induction of insolubility by herpes simplex virus vp22 precludes intercellular trafficking of n-terminal apoptin-vp22 fusion proteins. J Mol Med, 81(9): 558–565.
Saha S, Yoshida S, Ohba K, et al. 2006. A fused gene of nucleoprotein (np) and herpes simplex virus genes (vp22) induces highly protective immunity against different subtypes of influenza virus. Virology, 354(1): 48–57.
Schwarze S R, Hruska K A, Dowdy S F. 2000. Protein transduction: Unrestricted delivery into all cells? Trends Cell Biol, 10(7): 290–295.
Sciortino M T, Taddeo B, Poon A P, et al. 2002. Of the three tegument proteins that package mrna in herpes simplex virions, one (vp22) transports the mrna to uninfected cells for expression prior to viral infection. Proc Natl Acad Sci U S A, 99(12): 8318–8323.
Sciortino M T, Taddeo B, Giuffre-Cuculletto M, et al. 2007. Replication-competent herpes simplex virus 1 isolates selected from cells transfected with a bacterial artificial chromosome DNA lacking only the ul49 gene vary with respect to the defect in the ul41 gene encoding host shutoff rnase. J Virol, 81(20): 10924–10932.
Sellers J R. 2000. Myosins: A diverse superfamily. Biochim Biophys Acta, 1496(1): 3–22.
Seo S B, McNamara P, Heo S, et al. 2001. Regulation of histone acetylation and transcription by inhat, a human cellular complex containing the set oncoprotein. Cell, 104(1): 119–130.
Stroh C, Held J, Samraj A K, et al. 2003. Specific inhibition of transcription factor nf-kappab through in-tracellular protein delivery of i kappabalpha by the herpes virus protein vp22. Oncogene, 22(34): 5367–5373.
Taddeo B, Sciortino M T, Zhang W, et al. 2007. Interaction of herpes simplex virus rnase with vp16 and vp22 is required for the accumulation of the protein but not for accumulation of mrna. Proc Natl Acad Sci USA, 104(29): 12163–12168.
van Leeuwen H, Elliott G, O’Hare P. 2002. Evidence of a role for nonmuscle myosin ii in herpes simplex virus type 1 egress. J Virol, 76(7): 3471–3481.
van Leeuwen H, Okuwaki M, Hong R, et al. 2003. Herpes simplex virus type 1 tegument protein vp22 interacts with taf-i proteins and inhibits nucleosome assembly but not regulation of histone acetylation by inhat. J Gen Virol, 84(Pt 9): 2501–2510.
Vittone V, Diefenbach E, Triffett D, et al. 2005. Determination of interactions between tegument proteins of herpes simplex virus type 1. J Virol, 79(15): 9566–9571.
Wybranietz W A, Prinz F, Spiegel M, et al. 1999. Quantification of vp22-gfp spread by direct fluorescence in 15 commonly used cell lines. J Gene Med, 1(4): 265–274.
Xiong F, Xiao S, Yu M, et al. 2007. Enhanced effect of microdystrophin gene transfection by hsv-vp22 mediated intercellular protein transport. BMC Neurosci, 8: 50.
Xiong F, Xiao S, Peng F, et al. 2007. Herpes simplex virus vp22 enhances adenovirus-mediated microdystrophin gene transfer to skeletal muscles in dystrophin-deficient (mdx) mice. Hum Gene Ther, 18(6): 490–501.
Yu X, Li W, Liu L, et al. 2008. Functional analysis of transcriptional regulation of herpes simplex virus type 1 tegument protein vp22. Sci China C Life Sci, 51(11): 966–972.
Zhang Y, Sirko D A, McKnight J L. 1991. Role of herpes simplex virus type 1 ul46 and ul47 in alpha tif-mediated transcriptional induction: Characterization of three viral deletion mutants. J Virol, 65(2): 829–841.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation items: The Startup Fund of the Hundred Talents Program of the Chinese Academy of Science (20071010-141); National Natural Science Foundation of China (30870120); Open Research Fund Program of the State Key Laboratory of Virology of China (2007003, 2009007).
Equal contribution author.
Rights and permissions
About this article
Cite this article
Li, Ml., Guo, H., Ding, Q. et al. A multiple functional protein: the herpes simplex virus type 1 tegument protein VP22. Virol. Sin. 24, 153–161 (2009). https://doi.org/10.1007/s12250-009-3035-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12250-009-3035-2