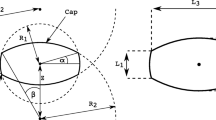
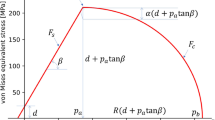
Abstract
Purpose
The objective of this work was to develop, validate, and implement a modeling methodology for predicting the shape and relative density fields in the vicinity of debossed features on a tablet surface. The resulting model was used to investigate the influence of debossed feature stroke angle and degree of pre-pick, which is expressed as a percentage of the stroke depth, as well as the influence of formulation lubricant on the aforementioned debossed feature parameters.
Methods
An experimental procedure for measuring formulation (modified) Drucker–Prager Cap parameters is described. These parameters are used in a finite element method simulation that models the formation of a debossed surface feature on a tablet. Techniques for validating the simulation and post-processing the results are also described.
Results
The stroke angle and degree of pre-pick significantly influence the debossed feature dimensions, with larger degrees of pre-pick and stroke angles giving debossed features that more closely match the target (embossment) values. Lubrication plays a much weaker role, but did improve the fidelity of the debossed feature slightly. The differences between the debossed and target feature dimensions are due to elastic spring back of the material. The tablet relative density is smallest at the shoulders of the debossed feature and largest at the base of the valley. Although the relative density fields show no obvious trends with stroke angle, the fields are clearly more uniform as the degree of pre-pick increases. The addition of lubricant to the formulation also improves the relative density field uniformity for larger degrees of pre-pick.
Conclusions
To improve feature fidelity and decrease the likelihood of damage, larger pre-picks, larger stroke angles, and the addition of a formulation lubricant should be used.



















Similar content being viewed by others
References
Manual of Policies and Procedures: Scoring Configuration of Generic Drug Products (5223.2). Center for Drug Evaluation and Research, Food and Drug Administration. May 2012. http://www.fda.gov/downloads/AboutFDA/ReportsManualsForms/StaffPoliciesandProcedures/ucm079779.pdf. Accessed 23rd June 2015.
Guidance for Industry Tablet Scoring: Nomenclature, Labeling, and Data for Evaluation. Center for Drug Evaluation and Research, Food and Drug Administration. March 2013. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm269921.pdf. Accessed 23rd June 2015.
McDermott TS, Farrenkopf J, Hlinak A, Neilly JP, Sauer D. A material sparing method for quantitatively measuring tablet sticking. Powder Technol. 2011;212(1):240–52.
Waimer F, Krumme M, Danz P, Tenter U, Schmidt PC. The influence of engravings on the sticking of tablets. Investigations with an instrumented upper punch. Pharm Dev Technol. 1999;4(3):369–75.
Sabir A, Evans B, Jain S. Formulation and process optimization to eliminate picking from market image tablets. Int J Pharm. 2001;215(1-2):123–35.
Armstrong N, Haines-Nutt R. Elastic recovery and surface area changes in compacted powder systems. Powder Technol. 1974;9(5-6):287–90.
Davies PN, Worthington HEC, Podczeck F, Newton JM. The determination of the mechanical strength of tablets of different shapes. Eur J Pharm Biopharm. 2007;67(1):268–76.
American Pharmaceutical Association Tableting Specification Steering Committee. Tableting specification manual. 7th ed. APhA Publications; 2005.
Pedersen M. Tablet tooling: design, maintenance and troubleshooting. Pharm Technol Eur. 1999;2:22–8.
Tsiftsoglou TB, Mendes RW. Effect of boron alloy coating of tableting tools. Pharm Technol. 1982;6:30–2.
Schumann S, Searle GD. The effect of chromium nitride ion bombardment treatment of tablet tooling on tablet adherence. Drug Dev Ind Pharm. 1992;18(10):1037–61.
Roberts M, Ford JL, MacLeod GS, Fell JT, Smith GW, Rowe PH. Effects of surface roughness and chrome plating of punch tips on the sticking tendencies of model ibuprofen formulations. J Pharm Pharmacol. 2003;55(9):1223–8.
Waimer F, Krumme M, Danz P, Tenter U, Schmidt PC. A novel method for the detection of sticking of tablets. Pharm Dev Technol. 1999;4(3):359–67.
Mullarney, Matthew P, Bruce C, MacDonald, Allan H. Assessing tablet-sticking propensity. Pharm Technol. 2012;36(1):57–62.
Laity PR. Effects of punches with embossed features on compaction behaviour. Powder Technol. 2014;254:373–86.
Helwany, S. Applied Soil Mechanics with ABAQUS Applications. 1st ed. Wiley; 2007.
Michrafy A, Ringenbacher D, Tchoreloff P. Modelling the compaction behaviour of powders: application to pharmaceutical powders. Powder Technol. 2002;127(3):257–66.
Sinka IC, Cunningham JC, Zavaliangos A. The effect of wall friction in the compaction of pharmaceutical tablets with curved faces: a validation study of the Drucker–Prager Cap model. Powder Technol. 2003;133(1):33–43.
Cunningham JC, Sinka IC, Zavaliangos A. Analysis of tablet compaction. I. Characterization of mechanical behavior of powder and powder/tooling friction. J Pharm Sci. 2004;93(8):2022–39.
Djemai A, Sinka IC. NMR imaging of density distributions in tablets. Int J Pharm. 2006;319(1):55–62.
Han LH, Elliott JA, Bentham AC, Mills A, Amidon GE, Hancock BC. A modified Drucker-Prager Cap model for die compaction simulation of pharmaceutical powders. Int J Solids Struct. 2008;45(10):3088–106.
Sinha T, Bharadwaj R, Curtis JS, Hancock BC, Wassgren C. Finite element analysis of pharmaceutical tablet compaction using a density dependent material plasticity model. Powder Technol. 2010;202(1):46–54.
Muliadi A, Litster JD, Wassgren C. Modeling the powder roll compaction process: comparison of 2-D finite element method and the rolling theory for granular solids (Johanson’s model). Powder Technol. 2012;221:90–100.
Drucker DC. Limit analysis of two and three dimensional soil mechanics problems. J Mech Phys Solids. 1953;1(4):217–26.
Schofield, Andrew, and Peter Wroth. Critical state soil mechanics. McGraw Hill; 1968.
Green RJ. A plasticity theory for porous solids. Int J Mech Sci. 1972;14(4):215–24.
DiMaggio FL, Ivan SS. Material model for granular soils. J Eng Mech. 1971;97(3):935–50.
Gurson AL. Continuum theory of ductile rupture by void nucleation and growth: Part I—Yield criteria and flow rules for porous ductile media. J Eng Mater Technol. 1977;99(1):2–15.
Kraft T, Riedel H. Numerical simulation of die compaction and sintering. Powder Metall. 2002;45(3):227–31.
Brewin P.R., Coube O., Doremus P., and Tweed J.H. Modelling of powder die compaction. Springer Science and Business Media; 2007.
Shang C, Sinka IC, Pan J. Constitutive model calibration for powder compaction using instrumented die testing. Exp Mech. 2012;52(7):903–16.
Chen WF, Baladi GY. Soil plasticity: theory and implementation. New York: Elseveir; 1985.
Teunou E, Fitzpatrick J. Effect of relative humidity and temperature on food powder flowability. J Food Eng. 1999;42:109–16.
Emery E, Oliver J, Pugsley T, Sharma J, Zhou J. Flowability of moist pharmaceutical powders. Powder Technol. 2009;189(3):409–15.
Amidon GE, Houghton ME. The effect of moisture on the mechanical and powder flow properties of microcrystalline cellulose. Pharm Res. 1995;12:923–9.
Fitzpatrick JJ, Iqbal T, Delaney C, Twomey T, Keogh MK. Effect of powder properties and storage conditions on the flowability of milk powders with different fat contents. J Food Eng. 2004;64(4):435–44.
Procopio A.T. On the Compaction of Granular Media Using a Multi-particle Finite Element Model. Ph.D. Thesis, Drexel University; 2006.
Awaji H, Sato S. Diametral compressive testing method. J Eng Mater Technol. 1979;101(2):139–47.
Busignies V, Leclerc B, Porion P, Evesque P, Couarraze G, Tchoreloff P. Quantitative measurements of localized density variations in cylindrical tablets using x-ray microtomography. Eur J Pharm Biopharm. 2006;64(1):38–50.
Sinka IC, Burch SF, Tweed JH, Cunningham JC. Measurement of density variations in tablets using x-ray computed tomography. Int J Pharm. 2004;271(1):215–24.
Dale S, Wassgren C, Litster J. Measuring granule phase volume distributions using x-ray microtomography. Powder Technol. 2014;264:550–60.
Hancock BC, Colvin JT, Mullarney MP, Zinchuk AV. The relative densities of pharmaceutical powders, blends, dry granulations, and immediate-release tablets. Pharm Technol. 2003;27:64–80.
Zuurman K, Van der Voort Maarschalk K, Bolhuis GK. Effect of magnesium stearate on bonding and porosity expansion of tablets produced from materials with different consolidation properties. Int J Pharm. 1999;179(1):107–15.
Wang J, Wen H, Desai D. Lubrication in tablet formulations. Eur J Pharm Biopharm. 2010;75(1):1–15.
Otsuka M, Yamane I, Matsuda Y. Effects of lubricant mixing on compression properties of various kinds of direct compression excipients and physical properties of the tablets. Adv Powder Technol. 2004;15(4):477–93.
S. Likitlersuang On the The Influence Of Magnesium Stearate On Pharmaceutical Powder Consolidation. Ph.D. Thesis, University of Iowa; 2004
Yu S, Adams M, Gururajan B, Reynolds G, Roberts R, Wu CY. The effects of lubrication on roll compaction, ribbon milling and tabletting. Chem Eng Sci. 2013;86:9–18.
LaMarche K, Buckley D, Hartley R, Qian F, Badawy S. Assessing materials’ tablet compaction properties using the Drucker–Prager Cap model. Powder Technol. 2014;267:208–20.
MathWorks. Image Processing Toolbox TM User’s Guide R2014b. Mathworks Pub; 2014.
Martin CL. Elasticity, fracture and yielding of cold compacted metal powders. J Mech Phys Solids. 2004;52(8):1691–717.
Briscoe BJ, Rough SL. The effects of wall friction in powder compaction. Colloids Surf A: Physicochemistry Eng Aspects. 1998;137(1-3):103–16.
Dawes J, Gamble JF, Greenwood R, Robbins P, Tobyn M. An investigation into the impact of magnesium stearate on powder feeding during roller compaction. Drug Dev Ind Pharm. 2012;38(1):111–22.
Acknowledgments
The authors would like to thank Chuck Kettler and Bill Turner from Natoli Engineering for their helpful suggestions and for providing the tool used in the experimental studies. The authors would also like to thank Eli Lilly and Company for providing funding to support this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Swaminathan, S., Hilden, J., Ramey, B. et al. Modeling the Formation of Debossed Features on a Pharmaceutical Tablet. J Pharm Innov 11, 214–230 (2016). https://doi.org/10.1007/s12247-016-9257-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-016-9257-6