Abstract
Background
There is an extensive critical care literature for central venous catheter and arterial line infection, duration of catheterization, and compliance with infection control procedures. The emergency medicine literature, however, contains very little data on central venous catheters and arterial lines. As emergency medicine practice continues to incorporate greater numbers of critical care procedures such as central venous catheter placement, infection control is becoming a greater issue.
Aims
We performed a systematic review of studies reporting baseline data of ED-placed central venous catheters and arterial lines using multiple search methods.
Methods
Two reviewers independently assessed included studies using explicit criteria, including the use of ED-placed invasive lines, the presence of central line-associated bloodstream infection, and excluded case reports and review articles. Finding significant heterogeneity among studies, we performed a qualitative assessment.
Results
Our search produced 504 abstracts, of which 15 studies were evaluated, and 4 studies were excluded because of quality issues leaving 11 cohort studies. Four studies calculated infection rates, ranging 0–24.1/1,000 catheter-days for central line-associated and 0–32.8/1,000 catheter-days for central line-related bloodstream infection. Average duration of catheterization was 4.9 days (range 1.6–14.1 days), and compliance with infection control procedures was 33–96.5%. The data were too poor to compare emergency department to in-hospital catheter infection rates.
Conclusions
The existing data for emergency department-placed invasive lines are poor, but suggest they are a source of infection, remain in place for a significant period of time, and that adherence to maximum barrier precautions is poor. Obtaining accurate rates of infection and comparison between emergency department and inpatient lines requires prospective study.
Similar content being viewed by others
Introduction
The study of central line-related and central line-associated bloodstream infection (CLRBSI and CLABSI, see Fig. 1) has a long history in the critical care setting [1–8], but largely excludes invasive lines (central venous and arterial catheters) placed in the emergency department (ED). While simple measures, such as a checklist of infection control procedures and observer, have been shown to eliminate invasive catheter infection in the intensive care unit [9], a paucity of such data exist in the ED.
Centers for Disease Control definitions for bloodstream and catheter infections [43]
Invasive catheter infections are a leading cause of hospital-acquired infection [10]. In the United States each year, roughly 80,000 CLRBSIs [1] occur in the intensive care unit, at a rate of 5.3/1,000 catheter days, with an attributable mortality up to 35%, and a cost of $34,508–$56,000 per episode of infection [5–8]. Hospital-wide, this amounts to as many as 250,000 cases annually, with an attributable mortality between 12–25% for each infection [11]. Recently the implementation of best practices to reduce line infections has become a national health care priority [12–14]. Changing patterns of care in the ED, including increasing visits, early goal-directed therapy for sepsis [15, 16], and greater boarding of admitted patients likely explain the rising number of invasive lines placed in the ED [17]. To our knowledge, the rate of CLRBSI and CLABSI from ED-placed invasive lines is not known. Therefore, we aimed to systematically review the literature to search for evidence of: (1) the rate of infection from invasive lines placed in the ED, (2) the duration of catheterization, and (3) process outcomes such as the use of infection control procedures.
Methods
We reported this study following guidelines set forth in the Cochrane Handbook for Systematic Reviews of Interventions [18].
Study strategy
We defined the search strategy and inclusion and exclusion criteria a priori. We aimed to identify studies that met one or more of the following inclusion criteria: (1) ED-placed invasive lines, (2) measured a quantitative (CLRBSI, CLABSI, bloodstream infection) or qualitative (catheter security, dressing condition and durability, skin condition and irritation) infectious outcome, duration of line placement, and infection control procedures, and (3) the study design included an analysis of cohort, case-control, cross-sectional, or experimental data. Exclusion criteria included: (1) no documented ED-placed invasive lines, (2) case series, case report, and review articles, (3) study of tunneled, Hickman, dialysis, and PICC lines, (4) no abstract or outcome data available, and (5) abstracts written in a language other than English or French.
Two reviewers (A.A. and C.L.), with the assistance of a medical research librarian, searched the entire MEDLINE (PubMed), EMBASE (EMBASE.com), and Cinahl (EBSCOhost) databases using a Boolean combination of the search terms: “emergency department,” “central venous line,” “arterial line,” and “infection” on June 19, 2008 (see Appendix A for full search terms). All terms were expanded in each database using keywords (MeSH and EMTREE terms, Cinahl headings) and plain text search terms. The searches were not limited by date of publication.
To avoid publication bias we explored the grey literature [19]. One reviewer (A.A.) searched conference abstracts available online from the past 5 years from two leading emergency medicine professional societies (American College of Emergency Physicians, 2003–2007; Society of Academic Emergency Medicine, 2006–2008 conference proceedings), the Conference Papers Index (1982–present), and contacted four major central venous catheter (CVC) and arterial line manufacturers (Arrow International/Teleflex, Cook Medical, Edwards Lifesciences, and Smiths Medical). We contacted members of the Institute for Healthcare Improvement Central Line Infection Mentor Hospital Registry [12] querying for unpublished data. We also snowballed (i.e., searched the references section of each included study to find further articles) included articles and contacted experts to obtain further references.
Study selection
One reviewer (A.A.) examined each of the abstracts returned by the search and screened for relevant articles to be reviewed in full. A second reviewer (C.L.) examined the first 50 abstracts, and agreement was calculated using the κ statistic. Articles that did not explicitly meet the study criteria based on the title and abstract were excluded. Any article that either met the study criteria or had an ambiguous or missing abstract was set aside for full review. Two reviewers (C.L and J.S.) independently performed a full article review of the remaining articles for inclusion and study quality, then discussed the articles until they reached consensus. We contacted authors of relevant articles when further data collection was necessary and excluded those that could not supply necessary data.
Outcome measurements and data analysis
Two reviewers (C.L., J.S.) independently assessed study quality using the Strengthening the Reporting of Observational Studies in Epidemiology statement [20], then discussed each study until they reached consensus. The quality criteria included validity and reliability of outcome definitions and measurements, and protection against bias. Studies with low risk of bias met all quality criteria for design and risk of bias, while those with moderate risk of bias met at least one criterion for design and risk of bias, and contained no fatal flaw that invalidated the results. Studies with high risk of bias lacked at least one important criterion for design and risk of bias that invalidated the results.
Data extraction
We designed data collection forms for the abstract-only and full-text article reviews (Appendix B), and collected the following elements from each study: design, setting (ED or not), sample size, type of line placed, number of lines placed, infectious outcomes (CLRBSI, CLABSI), catheter-days, and infection control procedures. We report the mean length of catheter placement and range, but individual line data were not available for calculating 95% confidence intervals. We also collected data on factors relating to study validity and reliability measures for cohort, case control, and experimental study designs.
Data synthesis
A high degree of study heterogeneity precludes internal subgroup analysis or meta-analysis.
Results
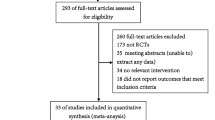
Figure 2 illustrates a flowchart of this systematic review. One reviewer (A.A.) examined each of the 504 abstracts returned by the search, screened for relevant articles, and obtained 84 articles for full review. Agreement between reviewers for abstracts was 100% (κ = 1). Two reviewers (C.L and J.S.) independently performed a full article review of the 84 remaining manuscripts, of which 69 articles did not meet selection criteria, leaving 15 articles that were assessed for quality. Four articles were excluded because of poor study quality [21–24], leaving 11 studies for inclusion. One study was excluded because it lacked sufficient data to evaluate its quality [21]. Two studies were excluded after contacting the authors because of insufficient data [23, 24], and one study was excluded for poor methodological quality [22]. A summary of the quality assessment for excluded and included studies is shown in Table 1 and Appendix C, respectively.
Table 2 provides characteristics of the 11 included papers [25–35]. These papers come from nine separate studies, since Koh [30] and Gowardman [29] are published from a single data set, and Guzzo [34] and Xiao [35] are also published from a single data set. Guzzo [34] and Xiao [35] measured CLRBSI, but a rate was not calculable because they did not measure catheter-days. Together the studies evaluated 523 (20.8%) ED-placed invasive lines from a total of 2,518 invasive lines. This amounted to 503 CVCs and 20 arterial lines (ALs) in the ED, and 2,195 CVCs and 323 ALs total, with all ALs from one study [35]. Six studies recorded both ED and inpatient CVCs, including those from the intensive care unit, ward, and operating room [26, 27, 29–33]. Three studies [25, 28, 34, 35] observed ED-placed CVCs exclusively, representing 313 (62.2%) of ED CVCs in this review. Citak's [26] was the only study to document whether ED CVCs were placed under emergent conditions, though only one of six emergent lines in their study was placed in the ED.
All nine studies reported ED infectious outcomes [25–35]. Five measured CLRBSI [7, 29, 30, 33–35] and four measured CLABSI [25, 28, 31, 32]. Six studies measured an infectious rate in the ED and elsewhere [26, 27, 29–33], though only Trick [32] analyzed the ED rate [23.5/1,000 catheter-days (95% CI 2.8–85), CLABSI] relative to the non-ED rate [1.68/1,000 catheter-days (95% CI 0.0–5.0), CLABSI]. They did not find this difference to be significant (P = 0.09), but the study had limited power with only 85 catheter-days total.
There were 1,837 (10.0%) ED and 18,409 total invasive line days, with 1,780 CVC and 57 AL days in the ED, and 17,327 CVC and 1,082 AL days in total [25–33]. The duration of CVCs ranged from 1.6 to 14.1 days for ED-placed CVCs and 1.6 to 18.9 days for all CVCs.
Four studies [26, 31, 33–35] measured compliance with a priori-defined infection control procedures, but none compared rates of compliance with maximum barrier precautions between the ED and inpatient wards. Overall, compliance with infection control procedures ranged from 33–96.5% for all lines. Definitions of infection control procedures and antisepsis varied. Three studies [31, 33–35] used maximum barrier precautions (cap, mask, sterile gown, sterile gloves, full sterile drape) as their standard, and one study [34, 35] measured use of 2% chlorhexidine antisepsis. Guzzo and Xiao videotaped and reviewed CVC placement in a trauma ED, reporting compliance rates of 33%–88% [34, 35]. They found that compliance decreased with seniority, with PGY 1–3 residents 90% compliant with maximum barrier precautions, and PGY 4 residents, fellows, and attendings only 61% compliant. The authors found that supervising physicians often used nothing more than sterile gloves when intervening to help junior residents during difficult CVC placements [34, 35].
Appendix C illustrates the methodological quality of the nine included studies. We found 6 studies to have a high risk of bias [25–28, 32, 33], three studies to have a moderate risk of bias [29, 31, 34, 35], and no studies to have a low risk of bias.
Study methods were mostly retrospective [25, 28] or not clearly defined [26, 27, 32, 33], though Guzzo [34] and Xiao [35] prospectively enrolled physicians and videotaped line placement. Three studies [29, 30, 32, 33] used a different methodology for identifying ED versus non-ED invasive lines. The definitions of infectious outcome varied. Dipietrantonio [27] defined CLRBSI as either (1) fever that resolved after line removal or (2) catheter tip and blood culture with the same organism AND not associated with some other cause, but did not meet CDC criteria. Ferguson [28] defined CLABSI as positive blood and catheter tip culture with clinical sepsis, but failed to include the organism or other potential sources of bacteremia, while Trick [32] defined CLABSI as sepsis plus positive culture, but did not specify whether this included blood culture, catheter tip culture, or both. Chiang [25] and Nagashima [31] defined CLABSI incompletely as positive blood culture, and Guzzo [34] and Xiao [35] had “five episodes of CLRBSI,” but this was not defined. The remaining three studies [26, 29, 30, 33] defined CLRBSI appropriately.
Attempts to address confounding were generally poor [25–32], with the exception of two studies [33–35]. Many studies included a favored catheter location (femoral [25, 31] or subclavian [26]), indication (for parenteral nutrition [33]), or healthier population [25], but did not account for this in their statistical analysis. Trick [32] measured confounding factors, such as obesity, anatomic location of line placement, and hospital department, but did not include these factors in their analysis. Risk of bias was high, particularly for compliance with infection control procedures, as this was often reported by the operator [27, 31] or assumed to be 100% a posteriori [26]. Guzzo [34] and Xiao [35] did address bias, having two researchers interpret each videotape for compliance with maximum barrier precautions, and a third acting as arbiter, but did not blind physicians to the purpose of the study.
In general, study power, methods of statistical analysis, the appropriate use of variables, and the inclusion and exclusion of subjects were poor, or little information on these subjects was available. However, two studies developed statistical methods a priori [29, 30, 33], and Guzzo [34] and Xiao [35] adequately powered their study for compliance with infection control procedures.
Discussion
To our knowledge, this is the first systematic review of ED-placed invasive lines. The 11 included studies [25–35] suggest that ED lines are a source of infection, remain for a substantial period of time, and there is evidence from one well-designed study [34, 35] that the rate of compliance with infection control procedures is poor.
Comparing measured rates of ED CVC infection across studies and with non-ED CVCs is not possible. Differing means of: (1) defining invasive line infection, (2) case identification of ED vs. non-ED catheter placement [26, 27, 29, 30, 32, 33], (3) measuring confounding factors like compliance with infection control procedures [26, 27, 29, 30, 32, 33], and (4) inadequate power [26, 27, 29–33] prohibit internally valid comparisons of ED vs. non-ED lines, and externally valid meta-analysis. Finally, only one study reported rates of infection from arterial lines, which reflects a lack of appreciation for ED arterial line infection. This well-designed study suggests that rates of arterial line and CVC infection may be comparable [30].
ED-placed invasive lines stayed in patients for nearly 1 week, on average, after admission. This is surprising given that infection rates are directly proportional to duration of catheterization [23, 36] and the general perception that ED invasive lines are placed in “crash” or code situations where full barrier precautions are not used and, therefore, are less sterile [22, 27]. Established guidelines advise changing invasive lines as soon as possible when full barrier precautions are not used [44]. Our finding suggests that either emergency departments do use full sterile precautions when placing some invasive lines or that inpatient providers are not following the established guidelines. Further research is needed to measure compliance with infection control procedures in the emergency department.
Reported compliance with maximum barrier precautions and antisepsis varied, but there appears to be substantial room for improvement. Most studies [26, 29–31, 33–35] relied upon self-reported rates of compliance, which are subject to overestimation bias. We believe that Guzzo [34] and Xiao’s [35] measured rate of compliance, 33%–88%, most accurately reflects US ED practice. Investigators videotaped invasive line placement and could identify all forms of poor infection control procedure, including those for which the practitioner was not aware. Even while being directly observed and videotaped, senior practitioners in the ED failed to follow basic infection control procedures [34, 35].
This systematic review reveals that ED-placed invasive line infections warrant greater attention. Given poor compliance with infection control procedures and prolonged duration of catheterization, ED infection rates are unlikely to be less than those reported by intensive care units. In our experience, most ED invasive lines are placed with sufficient time for precautions and thus are amenable to quality improvement initiatives to reduce invasive line infection. With rising ED visits, early goal-directed therapy for sepsis, and increased boarding of admitted patients in the ED, the importance of ED invasive line infection will only grow. Unfortunately, prospective surveillance of ED-placed invasive lines is methodologically difficult. Prospective studies of sterility and infection require direct observation of line placement in the ED, inpatient follow-up for line removal, and infectious surveillance. Such collaborative work between the ED and inpatient teams can be difficult to coordinate.
Recent developments, including increased public focus on hospital-acquired infections and Medicare’s decision to end payments for invasive line infection during inpatient hospitalizations [37], will prompt changes in ED line placement. Most directly, the Joint Commission’s 2009 National Patient Safety Goals will require use of an invasive line checklist and observer in all areas of the hospital, a technique that is inexpensive, feasible to implement, and highly effective [9, 13, 14]. However, without published ED data, ED directors and administrators may have difficulty motivating staff to alter their practice or sustain change.
Limitations
Although systematic, our review has several limitations. First, it is impossible to be certain that we obtained all relevant articles. We developed our search with a research librarian with expertise in systematic reviews, used broad search terms, and attempted to search all relevant sources, including multiple databases and the grey literature. We also pulled full articles for review when we could not clearly exclude articles by title and abstract alone.
Second, scant reporting of findings from ED-placed CVCs and ALs, and the heterogeneous set of outcomes and study settings preclude meaningful summary statistics and comparative analysis. We systematically requested additional data from authors, but we were unable to contact all authors. Three studies, comprising 63% of ED catheter-days in this review, come from Japan [31] and Turkey [26, 33], where ED practice patterns differ from that of the US, including ED length of stay [38, 39], ED volume [38–42], and acute care training [38–42]. Chiang [25] and Citak [26] analyzed pediatric populations alone, while Yilmaz [33] looked exclusively at CVCs used for parenteral nutrition. Included studies spanned 22 years (1986–2008), during which time physicians abolished the practice of routinely changing CVCs over a wire and began using chlorhexidine. Therefore, it is difficult to generalize the results from multiple studies [25–28, 31, 33] to the current North American ED population.
Third, while we found data on ED rates of invasive line infection, this was not the a priori outcome for any of the studies we included.
Conclusions
This systematic review demonstrates a lack of high quality data for ED-placed invasive lines in the literature. While the available studies have flaws, they do suggest that ED invasive lines are a source of infection, remain in place for a substantial length of time, and that adherence to maximum barrier precautions is poor. We hope that future, prospective analysis of ED invasive lines will measure rates of infection and compare these rates to inpatient invasive lines.
References
National nosocomial infections surveillance (NNIS) system report (1998) data summary from October 1986–April 1998, issued June 1998. Am J Infect Control 26:522–533
Soufir L, Timsit JF, Mahe C, Carlet J, Regnier B, Chevret S (1999) Attributable morbidity and mortality of catheter-related septicemia in critically ill patients: A matched, risk-adjusted, cohort study. Infect Control Hosp Epidemiol 20:396–401
Digiovine B, Chenoweth C, Watts C, Higgins M (1999) The attributable mortality and costs of primary nosocomial bloodstream infections in the intensive care unit. Am J Respir Crit Care Med 160:976–981
Mermel LA (2000) Prevention of intravascular catheter-related infections. Ann Intern Med 132:391–402
Dimick JB, Pelz RK, Consunji R, Swoboda SM, Hendrix CW, Lipsett PA (2001) Increased resource use associated with catheter-related bloodstream infection in the surgical intensive care unit. Arch Surg 136:229–234
Rello J, Ochagavia A, Sabanes E, Roque M, Mariscal D, Reynaga E et al (2000) Evaluation of outcome of intravenous catheter-related infections in critically ill patients. Am J Respir Crit Care Med 162:1027–1030
Pittet D, Tarara D, Wenzel RP (1994) Nosocomial bloodstream infection in critically ill patients. Excess length of stay, extra costs, and attributable mortality. JAMA 271:1598–1601
Collignon PJ (1994) Intravascular catheter associated sepsis: A common problem. the Australian study on intravascular catheter associated sepsis. Med J Aust 161:374–378
Pronovost P, Needham D, Berenholtz S, Sinopoli D, Chu H, Cosgrove S et al (2006) An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med 355:2725–2732
Burke JP (2003) Infection control—a problem for patient safety. N Engl J Med 348:651–656
Kluger DM, Maki DG (1999) The relative risk of intravascular device related bloodstream infections in adults [abstract]. Abstracts of the 39th Interscience Conference on Antimicrobial Agents and Chemotherapy. Abstract 1913
The Institute for Healthcare Improvement 5 Million Lives Campaign: Prevent Central Line-Associated Bloodstream Infections. The Institute for Healthcare Improvement Website. Available at: http://www.ihi.org/IHI/Programs/Campaign/CentralLineInfection.htm. Accessed April 3, 2009.
Joint Commission (2007) The joint commission announces the 2008 national patient safety goals and requirements. Joint Comm Perspect 27:9–22
Implement best practices or evidence-based guidelines to prevent central line–associated bloodstream infections. In: The Joint Commission on Accreditation of Healthcare Organizations, National Patient Safety Goals for Hospitals. The Joint Commission on Accreditation of Healthcare Organizations; 2008:15–16. Available at: http://www.jointcommission.org/NR/rdonlyres/31666E86-E7F4-423E-9BE8-F05BD1CB0AA8/0/HAP_NPSG.pdf. Accessed April 3, 2009.
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B et al (2001) Early goal-directed therapy in the treatment of severe sepsis and septic shock. New Engl J Med 345:1368–1377
Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R et al (2008) Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 36:296–327
Glickman S, Krubert C, Koppenhaver J, Glickman L, Schulman K, Cairns C (2010) Increased rate of central venous catheterization procedures in community EDs. Am J Emerg Med 28:208–212
Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. Available at: www.cochrane-handbook.org. Accessed April 3, 2009.
Benzies KM, Premji S, Hayden KA, Serrett K (2006) State-of-the-evidence reviews: Advantages and challenges of including grey literature. Worldviews Evid Based Nurs 3:55–61
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP et al (2008) The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. J Clin Epidemiol 61:344–349
Balls A, LoVecchio F, Stapczynski S (2007) Central line emergency access registry: A multi-center study to determine resident competency with central venous catheter insertion. research forum abstracts. Ann Emerg Med 50:S115
Pujol M, Hornero A, Saballs M, Argerich MJ, Verdaguer R, Cisnal M et al (2007) Clinical epidemiology and outcomes of peripheral venous catheter-related bloodstream infections at a university-affiliated hospital. J Hosp Infect 67:22–29
Collin GR (1999) Decreasing catheter colonization through the use of an antiseptic-impregnated catheter: A continuous quality improvement project. Chest 115:1632–1640
Jamulitrat S, Narong MN, Thongpiyapoom S (2002) Trauma severity scoring systems as predictors of nosocomial infection. Infect Control Hosp Epidemiol 23:268–273
Chiang VW, Baskin MN (2000) Uses and complications of central venous catheters inserted in a pediatric emergency department. Pediatr Emerg Care 16:230–232
Citak A, Karabocuoglu M, Ucsel R, Uzel N (2002) Central venous catheters in pediatric patients–subclavian venous approach as the first choice. Pediatr Int 44:83–86
Dipietrantonio PJ (1986) Incidence of infection from central venous catheterization in a military community hospital. J Am Osteopath Assoc 86:158–161
Ferguson M, Max MH, Marshall W (1988) Emergency department infraclavicular subclavian vein catheterization in patients with multiple injuries and burns. South Med J 81:433–435
Gowardman JR, Robertson IK, Parkes S, Rickard CM (2008) Influence of insertion site on central venous catheter colonization and bloodstream infection rates. Intensive Care Med 34 (6):1038–1045
Koh DB, Gowardman JR, Rickard CM, Robertson IK, Brown A (2008) Prospective study of peripheral arterial catheter infection and comparison with concurrently sited central venous catheters. Crit Care Med 36:397–402
Nagashima G, Kikuchi T, Tsuyuzaki H, Kawano R, Tanaka H, Nemoto H et al (2006) To reduce catheter-related bloodstream infections: Is the subclavian route better than the jugular route for central venous catheterization? J Infect Chemother 12:363–365
Trick WE, Miranda J, Evans AT, Charles-Damte M, Reilly BM, Clarke P (2006) Prospective cohort study of central venous catheters among internal medicine ward patients. Am J Infect Control 34:636–641
Yilmaz G, Koksal I, Aydin K, Caylan R, Sucu N, Aksoy F (2007) Risk factors of catheter-related bloodstream infections in parenteral nutrition catheterization. JPEN J Parenter Enteral Nutr 31:284–287
Guzzo JL, Seagull FJ, Bochicchio GV, Sisley A, Mackenzie CF, Dutton T et al (2006) Mentors decrease compliance with best sterile practices during central venous catheter placement in the trauma resuscitation unit. Surg Infect 7:15–20
Xiao Y, Seagull FJ, Bochicchio GV, Guzzo JL, Dutton RP, Sisley M et al (2007) Video-based training increases sterile-technique compliance during central venous catheter insertion. Crit Care Med 35:1302–1306
Raad I, Hanna H, Jiang Y, Dvorak T, Reitzel R, Chaiban G et al (2007) Comparative activities of daptomycin, linezolid, and tigecycline against catheter-related methicillin-resistant staphylococcus bacteremic isolates embedded in biofilm. Antimicrob Agents Chemother 51:1656–1660
Hospital-acquired conditions. United States Department of Health and Human Services: Centers for Medicare and Medicaid Services Website. Available at: http://www.cms.hhs.gov/HospitalAcqCond/06_Hospital-Acquired_Conditions.asp. Accessed April 3, 2009.
Lewin MR, Hori S, Aikawa N (2005) Emergency medical services in Japan: An opportunity for the rational development of pre-hospital care and research. J Emerg Med 28:237–241
Rinnert SM, Miller ACM, Murat EMP (2008) International emergency medicine: The Turkish experience. Common sense. Am Acad Emerg Med 15:11
Ezaki T, Yamada T, Yasuda M, Kanna T, Shiraishi K, Hashizume M (2007) Current status of Japanese emergency medicine based on a cross-sectional survey of one prefecture. Emerg Med Australas 19:523–527
Ezaki T, Hashizume M (2007) Emergency medicine in Japan. A look at a current university hospital and the problems faced. Emerg Med Australas 19:296–299
O'Malley RN, O'Malley GF, Ochi G (2001) Emergency medicine in Japan. Ann Emerg Med 38:441–446
Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF (1999) Improving the quality of reports of meta-analyses of randomised controlled trials: The QUOROM statement. Quality of reporting of meta-analyses. Lancet 354:1896–1900
O'Grady NP, Alexander M, Dellinger EP et al (2002) Guidelines for the prevention of intravascular catheter-related infections. centers for disease control and prevention. MMWR Recomm Rep 51:1–29
Acknowledgment
Supported by a Richard C. Wuerz Research Grant, from the Department of Emergency Medicine, Brigham and Women’s Hospital, Boston, MA, USA.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
The views expressed in this paper are those of the author(s) and not those of the editors, editorial board or publisher.
Appendices
Appendix A. Detailed search strategy
PubMed (MEDLINE) A-line search
("Emergency Service, Hospital"[Mesh] OR "Emergency Medicine"[Mesh] OR "emergencies"[MeSH Terms] OR "Emergency treatment"[Mesh] OR "emergency medical services"[mesh] OR "emergency department"[All Fields] OR "emergency departments"[All Fields] OR ((emergenc*[tiab] NOT Medline[SB]) AND department*[tiab]) OR "emergency room"[All Fields] OR "emergency rooms"[All Fields] OR "emergency ward"[All Fields] OR "emergency wards"[All Fields] OR "ED"[All Fields] OR "ER"[All Fields] OR "EW"[All Fields]) AND ("Catheterization, Peripheral"[Mesh] OR "Catheters, Indwelling"[Mesh] OR "Indwelling Catheter"[All Fields] OR "Indwelling Catheters"[All Fields] OR "In-Dwelling Catheters"[All Fields] OR "In Dwelling Catheters"[All Fields] OR "In-Dwelling Catheter"[All Fields] OR "Intra Arterial Lines"[All Fields] OR "Intra Arterial Lines"[All Fields] OR "Intra-Arterial Line"[All Fields] OR "Arterial Lines"[All Fields] OR "Arterial Line"[All Fields] OR "arterial catheter" OR "arterial catheters") AND ("Infection"[Mesh] OR "infectio*" OR "septic" OR "sepsis" OR "bacteremia" OR "bacteremias" OR "blood culture" OR "mycosis" OR "mycoses" OR "fungemia" OR "fungemias")
PubMed (MEDLINE) CVC search
("Emergency Service, Hospital"[Mesh] OR "Emergency Medicine"[Mesh] OR "emergencies"[MeSH Terms] OR "Emergency treatment"[Mesh] OR "emergency medical services"[mesh] OR "emergency department"[All Fields] OR "emergency departments"[All Fields] OR ((emergenc*[tiab] NOT Medline[SB]) AND department*[tiab]) OR "emergency room"[All Fields] OR "emergency rooms"[All Fields] OR "emergency ward"[All Fields] OR "emergency wards"[All Fields] OR "ED"[All Fields] OR "ER"[All Fields] OR "EW"[All Fields]) AND ("Catheterization, Central Venous"[Mesh] OR ("central" AND (catheter* OR cannul* OR "line" OR "access") AND ("vein" OR "venous")) OR "cvc" OR "cvl") AND ("Infection"[Mesh] OR "infectio*" OR "septic" OR "sepsis" OR "bacteremia" OR "bacteremias" OR "blood culture" OR "mycosis" OR "mycoses" OR "fungemia" OR "fungemias")
EMBASE A-line search
('Emergency health service'/exp OR 'emergency medicine'/exp OR 'emergency'/exp OR 'emergency treatment'/exp OR 'emergency department' OR 'emergency departments' OR (emergenc*:ab,ti AND department*:ab,ti) OR 'emergency room' OR 'emergency rooms' OR 'emergency ward' OR 'emergency wards' OR 'ED' OR 'ER' OR 'EW') AND ('infection'/exp OR 'infection' OR 'infections' OR 'septic' OR 'sepsis' OR 'bacteremia' OR 'bacteremias' OR 'blood culture' OR 'mycosis' OR 'mycoses' OR 'fungemia' OR 'fungemias') AND ('artery catheterization'/exp OR 'artery catheter'/exp OR 'lung artery catheter'/exp OR 'pulmonary artery catheter'/exp OR 'Swan Ganz catheter'/exp OR 'Indwelling Catheter' OR 'Indwelling Catheters' OR 'In-Dwelling Catheters' OR 'In Dwelling Catheters' OR 'In-Dwelling Catheter' OR 'Intra Arterial Lines' OR 'Intra Arterial Lines' OR 'Intra-Arterial Line' OR 'Arterial Lines' OR 'Arterial Line' OR 'arterial catheter' OR 'arterial catheters' OR 'swan ganz')
EMBASE CVC search
('Emergency health service'/exp OR 'emergency medicine'/exp OR 'emergency'/exp OR 'emergency treatment'/exp OR 'emergency department' OR 'emergency departments' OR (emergenc*:ab,ti AND department*:ab,ti) OR 'emergency room' OR 'emergency rooms' OR 'emergency ward' OR 'emergency wards' OR 'ED' OR 'ER' OR 'EW') AND ('infection'/exp OR 'infection' OR 'infections' OR 'septic' OR 'sepsis' OR 'bacteremia' OR 'bacteremias' OR 'blood culture' OR 'mycosis' OR 'mycoses' OR 'fungemia' OR 'fungemias') AND ('central venous catheterization'/exp OR 'central venous catheter'/exp OR ('central' AND (catheter* OR cannul* OR 'line' OR 'access') AND ('vein' OR 'venous')) OR 'cvc' OR 'cvl')
Cinahl A-line search
(MH "Emergency Medical Services + " OR MH "Emergency Medicine" OR MH "Emergency Service + " OR MH "Emergencies + " OR MH "Emergency Care + " OR MH "Emergency Treatment (Non-Cinahl) + " OR "emergency department" OR "emergency departments" OR "emergency room" OR "emergency rooms" OR "emergency ward" OR "emergency wards") AND (MH "Infection + " OR "infection" OR "infections" OR "septic" OR "sepsis" OR "bacteremia" OR "bacteremias" OR "blood culture" OR "mycosis" OR "mycoses" OR "fungemia" OR "fungemias") AND (MH "Catheterization, Peripheral + " OR MH "Arterial Catheters" OR MH "Pulmonary Artery Catheters + " OR "Indwelling Catheter" OR "Indwelling Catheters" OR "In-Dwelling Catheters" OR "In Dwelling Catheters" OR "In-Dwelling Catheter" OR "Intra Arterial Lines" OR "Intra Arterial Lines" OR "Intra-Arterial Line" OR "Arterial Lines" OR "Arterial Line" OR "arterial catheter" OR "arterial catheters" OR "swan ganz")
Cinahl CVC search
(MH "Emergency Medical Services + " OR MH "Emergency Medicine" OR MH "Emergency Service + " OR MH "Emergencies + " OR MH "Emergency Care + " OR MH "Emergency Treatment (Non-Cinahl) + " OR "emergency department" OR "emergency departments" OR "emergency room" OR "emergency rooms" OR "emergency ward" OR "emergency wards") AND (MH "Infection + " OR "infection" OR "infections" OR "septic" OR "sepsis" OR "bacteremia" OR "bacteremias" OR "blood culture" OR "mycosis" OR "mycoses" OR "fungemia" OR "fungemias") AND (MH "Catheterization, Central Venous + " OR MH "Central Venous Catheters + " OR ("central" AND (catheter* OR cannul* OR "line" OR "access") AND ("vein" OR "venous")) OR "cvc" OR "cvl")
Conference papers index search
(Central Venous Catheter OR Arterial Line) AND (Infection OR Sepsis) AND (Emergenc* OR ED OR ER). The search was limited to the Natural Sciences Index and articles in English.
Google search
(Central Venous Catheter OR Arterial Line) AND (Infection) AND (Emergency Department). The search was limited to hits in the biology and medicine category.
Appendix B. Abstract selection form

Appendix B. Study appraisal form


Appendix C. Quality assessment of included studies
Table 3
Included study | Design | Patient selection | Well-defined outcome | Protection Against Confounding | Protection against bias | Adequate power | Proper variables | Proper statistical methods | Reported included and excluded subjects | Global assessment | Global assessment: emergency department vs. non-emergency department infection rate |
|---|---|---|---|---|---|---|---|---|---|---|---|
Dipietrantonio 1986 [27] | Retrospective chart review | Poor quality | Poor quality | Poor quality | Poor quality | Poor quality | Poor quality | Poor quality | Poor quality | High risk of bias | High risk of bias |
Ferguson 1988 [28] | Retrospective chart review | Poor quality | Good quality | Poor quality | Poor quality | Poor quality | Poor quality | Poor quality | Not done | High risk of bias | N/A |
Chiang 2000 [25] | Retrospective chart review | Poor quality | Poor quality | Poor quality | Poor quality | Poor quality | Poor quality | Poor quality | Not done | High risk of bias | N/A |
Citak 2002 [26] | Prospective observational cohort | Unclear | Good quality | Poor quality | Poor quality | Poor quality | Poor quality | Poor quality | Poor quality | High risk of bias | High risk of bias |
Nagashima 2006 [31] | Retrospective chart review | Good quality | Poor quality | Poor quality | Poor quality | Unclear | Poor quality | Poor quality | Poor quality | Moderate risk of bias | Moderate risk of bias |
Trick 2006 [32] | Prospective observational cohort | Poor quality | Poor quality | Poor quality | Poor quality | Poor quality | Poor quality | Poor quality | Poor quality | High risk of bias | High risk of bias |
Prospective observational cohort | Unclear | Good quality | Good quality | Poor quality | Good quality | Good quality | Poor quality | Not done | Moderate risk of bias | N/A | |
Yilmaz 2007 [33] | Prospective observational cohort | Unclear | Good quality | Good quality | Poor quality | Poor quality | Poor quality | Good quality | Not done | High risk of bias | High risk of bias |
Prospective observational cohort | Poor quality | Good quality | Poor quality | Poor quality | Poor quality | Good quality | Good quality | Good quality | Moderate risk of bias | High risk of bias |
Adapted from STROBE Statement [19]
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
LeMaster, C.H., Agrawal, A.T., Hou, P. et al. Systematic review of emergency department central venous and arterial catheter infection. Int J Emerg Med 3, 409–423 (2010). https://doi.org/10.1007/s12245-010-0225-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12245-010-0225-5