Abstract
The Atlantic ribbed mussel, Geukensia demissa, is found in salt marshes along the North American Atlantic Coast. As a first step to study the possibility of future cultivation and harvest of ribbed mussels for nutrient removal from eutrophic urban environments, the feeding behavior of ribbed mussels in situ was studied from July to October 2011. Two locations approximately 80 km apart were used as study sites: Milford Harbor (Connecticut; 41°12′42.46″N, 73°3′7.75″W) and Hunts Point (Bronx, New York; 40°48′5.99″N, 73°52′17.76″W). Total particulate matter was higher at Hunts Point than at Milford Harbor, but the organic content was higher at Milford than at Hunts Point. The relatively low quantity of organic content in Hunts Point seston resulted in a much higher production of pseudofeces by mussels. Mussel clearance and absorption rates were higher at Milford Harbor than at Hunts Point. Nevertheless, mussels at both sites had the same absorption efficiency, suggesting that mussels are able to adapt to conditions at both locations. Ribbed mussels decreased clearance rate when the seston quantity was high at both sites. At Hunts Point, ribbed mussels increased the gut transit time of ingested particles when the amount of inorganic particulates in the water increased. This study does not quantify nutrient removal capacity of G. demissa; however, the environmental tolerance demonstrated here, and current lack of commercial harvest, suggests that this species may be a good candidate for nutrient bioextraction in highly impacted urban environments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Atlantic ribbed mussel, Geukensia demissa, is found in intertidal habitats, mainly salt marshes, along the North American Atlantic Coast (Abbott 1974). Ribbed mussels feed on a wide range of particles suspended in the water column, including phytoplankton, bacteria, and detritus; mussels may also absorb dissolved organic material (Alber and Valiela 1994; Kreeger and Newell 1996a). Ribbed mussel gills can retain particles within a defined size range; particles >4 μm can be retained by G. demissa with 100 % efficiency (Riisgård 1988), although the detailed morphology of the ribbed mussel gill makes this species a very effective bacterial grazer in comparison to other bivalve species (Wright et al. 1982; Riisgård 1988; Langdon and Newell 1990; Kreeger and Newell 1996b). Ribbed mussels have a high ability to feed efficiently on different particles and exploit available resources, e.g., Peterson et al. (1985) reported that G. demissa consumed both Spartina alterniflora (marsh grass) detritus and plankton, with the relative proportions of each determined by the location of the mussels in the salt marsh. Accordingly, Kreeger and Newell (2001) concluded that the digestive physiology of ribbed mussels responds to shifts in dietary components during the year, suggesting that mussels efficiently balance nutritional demands according to food availability.
Ribbed mussels have unusual tolerance to dehydration, salinity variation, and thermal stress (Lent 1969). Moreover, because they inhabit sulfide-rich coastal sediments (Lee et al. 1996), ribbed mussels have much higher tolerance to sulfide than other bivalves (Doeller et al. 2001). These adaptations enable this mussel to be the dominant, benthic suspension feeder in salt marshes (Wright et al. 1982). Even though ribbed mussels are not commercially harvested, G. demissa plays a key role in the flow of nutrients in the ecosystems it inhabits, as observed in Delaware Bay (Kreeger et al. 2011). Jordan and Valiela (1982) found that ribbed mussels in a New England salt marsh filtered 1.8 times as much particulate nitrogen as was exported from the salt marsh and hypothesized that retention of nitrogen by mussels may enhance the productivity of the marsh. This ability to retain nitrogen also has the potential to mitigate eutrophication, defined by Nixon (1995) as an increase in the rate of supply of organic matter to an ecosystem that is related to human activities, thereby improving water quality (Ostroumov 2005; Coen et al. 2007; Lindahl and Kollberg 2009; Manganaro et al. 2009). The loss of bivalves and their ecological services in coastal and estuarine ecosystems can result in the same ecological effects as eutrophication (Kemp et al. 2005; Heck and Valentine 2007).
To remediate nutrient over-enrichment and enhance the quality of coastal waters, aquaculture of blue mussels (Mytilus edulis) has been used as a successful management tool to compensate for nutrient discharges in Sweden (Lindahl 2011). In the USA, this approach has been proposed as a nutrient reduction strategy in combination with point source reductions, such as wastewater treatment plant upgrades (Rose et al. 2010). The use of shellfish aquaculture to lower quantities of nutrients in the coastal environment is thought to be economically favorable when compared to other non-point, nutrient reduction measures such as agricultural best management practices or urban stormwater retrofitting (Hart 2003; Stephenson et al. 2010). The environmental management strategy by which nutrients are removed from an aquatic ecosystem through the harvest of enhanced biological production, including the aquaculture of suspension-feeding shellfish or algae, is being called nutrient bioextraction.
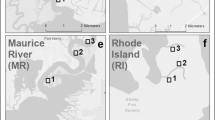
In the present study, in situ, filter-feeding measurements were conducted in two very different environments, Milford Harbor (Connecticut) and Hunts Point (Bronx, New York), to gain a better understanding of the feeding behavior of ribbed mussels and evaluate the potential use of ribbed mussel aquaculture as a nutrient bioextraction (Rose et al. 2010) tool. Both study sites support naturally occurring ribbed mussel populations but are very different in terms of physics, chemistry, and biology. Hunts Point is in a highly urban, densely populated, and industrial section of New York City, at the confluence of the Bronx River and the East River tidal strait. It is in close proximity to one of New York City’s largest wastewater treatment plants (the Hunts Point Wastewater Treatment Plant, which releases 378–757 × 106 L day−1 of tertiary treated effluent). In contrast, Milford Harbor is in suburban, coastal Connecticut, with a lower human population density, less industry, and much less riverine influence. The intent of this study was to determine if seston organic content at Hunts Point was sufficient to support cultivation and harvest of large populations of ribbed mussels, which would support their potential future use for nutrient removal in this environment. These two sites also provided a good test of the flexibility of ribbed mussel filtration effectiveness.
Methods
Experimental Design
Filter-feeding measurements were conducted from July 2011 to October 2011 at Milford Harbor, Connecticut (41°12′42.46″N, 73°3′7.75″W) and at Hunts Point, Bronx, New York (40°48′5.99″N, 73°52′17.76″W) (ESM Appendix 1), during different tidal cycles. Seven determinations were made at Milford and six at Hunts Point. Two portable, flow-through devices designed to quantify mussel feeding on natural seston (Galimany et al. 2011) were used. A brief description of one portable, filter-feeding device follows: a PVC tank received seawater from an underwater pump suspended at 1 m depth. A PVC “reservoir” tank was aerated to maintain suspension of particles in the water. Seawater flowed from the lower part of the reservoir tank through ten tubes, each connected to an individual, flow-through chamber. Each chamber contained a single mussel, except for two chambers which contained one empty mussel shell each used as controls. A thorough description of the design and operation of the units is available in Galimany et al. (2011).
Mussel shell length and dry weight were measured for each sampling day. Each filter-feeding measurement was conducted with a different group of similarly sized ribbed mussels (G. demissa) collected at the respective sites. Average shell lengths were 62.55 ± 9.07 and 60.95 ± 5.82 mm for Milford Harbor and Hunts Point, respectively. Mussels from both sites were collected 1 week prior to the experimental season and suspended in mesh bags at each sampling site for the duration of the season. Previously, we showed that tidal effects upon filtration behavior of ribbed mussels were no longer detected 7 days after the initiation of continuous submersion (Galimany et al. 2012). Mussels for the Milford Harbor determinations were collected from the Milford fringe salt marsh directly adjacent to the study site. Mussels for the Hunts Point measurements were collected from a fringe salt marsh at the mouth of the Bronx River, the closest marsh to Hunts Point. Sixteen mussels were selected randomly from the suspended mesh bag for each experiment, and epiphytes and other encrusting organisms were removed from the shells. A small, plastic, Velcro fastener was glued to one of the two shells of each individual and used to affix the mussel to the experimental chamber. Each mussel was exposed to a constant flow rate of 12 L h−1 of ambient water; this flux was shown in previous laboratory experiments to result in homogeneous distribution of particles between chambers with no water recirculation or lateral flow between the chambers. Mussels were allowed to recover for 2 h from any stress associated with handling, but acclimation was not necessary because the mussels had been submerged in ambient seawater prior to being installed within the apparatus, which was brought to the site. Four control, empty mussel shells, two per each feeding apparatus, were affixed in the chambers in the same manner as the live mussels (Galimany et al. 2011).
Seawater
Water (300 mL) was collected from the device intake and flow exit tube of each control feeding chamber every 15 min. The feces and pseudofeces from each live mussel were collected separately throughout the experiment with a pipette. All samples (water, feces and pseudofeces) were filtered separately through washed, pre-weighed Whatman GF/C filters (25 mm) and rinsed with isotonic ammonium formate. GF/C filters were used instead of GF/F filters because GF/C is the standard filter used in biodeposition experiments with mussels (Iglesias et al. 1998). We decided to maintain a consistent filter pore size in these studies to facilitate the comparison of our results with previous studies on other mussel species. Filters were kept on ice and transferred to the laboratory where they were dried at 60 °C for 48 h and weighed to obtain the dry weight, a measure of total particulate matter (TPM). Filters then were ashed at 450 °C for 4 h and weighed again to obtain the particulate inorganic matter (PIM). The particulate organic matter (POM) was calculated as the difference between TPM and PIM. The organic content of the water (f) was calculated as the mean organic fraction of total particulates (f = POM / TPM).
Physiological Feeding Variables
The individual chambers were cleaned before the beginning of the measurement period. All of the feces and pseudofeces from each individual mussel then were collected separately with a pipette as soon as they were produced to ensure that all biodeposits were included in quantitative analyses. The time period of collection of biodeposits (between 1 and 2 h) ensured collection of sufficient biodeposits by the end the experiment for accurate quantification. Feces and pseudofeces produced by each mussel (n = 16) were filtered individually and processed for organic and inorganic matter measurements as described above to compute the total, organic, and inorganic rates of egestion and rejection, respectively. Data from the few mussels that produced no feces or pseudofeces (i.e., did not open) during the measurement period were not included in subsequent analyses. The physiological components of the absorptive balance (Table 1) then were calculated according to the biodeposition method (Iglesias et al. 1998). To quantify pre-ingestive selection of food through pseudofeces production, we expressed rejection as a percentage of total filtered seston.
To synchronize the seston available with the corresponding biodeposits produced by the mussels, it was necessary to estimate the gut transit time (GTT). Gut transit time is defined as the minimum time for an organic particle to pass through the digestive tract of a mussel after ingestion. This variable was calculated before each measurement period using a method adapted from Hawkins et al. (1996). Five ribbed mussels, from the same suspended mesh bag, were placed in individual beakers containing a mixture of local seawater and cultured Tetraselmis chui (PLY 429). The time that elapsed between the addition of cultured T. chui and the first deposition of green-colored feces by one of the five mussels was considered to be the gut transit time (in minute).
All mussel variables were standardized to 1 g of dried mussel flesh using the following equation:
where, for the physiological variables, Y s is the standardized physiological rate, Y e is the experimentally determined rate, and W e is the dry body mass measured for each mussel. We used a b value of 0.83 as determined by Riisgård (1988) for G. demissa. For GTT, Y s is the standardized GTT, Y e is the experimentally determined GTT, and W e is the dry body mass measured for each mussel. We used a b value of 0.34, as determined by Hawkins et al. (1990) for blue mussels M. edulis because there is not a value for ribbed mussels in the literature.
Statistical Analyses
Statistical comparisons of the Hunts Point to the Milford Harbor mussels and water were performed using a percentile bootstrap test for trimmed means as described by Wilcox (2003). This method was chosen over the commonly used Student’s t test for comparing two independent groups because the percentile bootstrap test has higher power and requires no assumptions of normality or heteroscedasticity. Correlations were established between the organic content of the water (f) and total particulate matter, rejection proportion and total particulate matter, rejection proportion and organic content of the ingested matter (i), clearance rate and total particulate matter, and between standard gut transit time and total particulate matter. Separate correlations were established for each site using the percentage bend correlation, which is a robust analog of the Pearson correlation described in detail by Wilcox (2003). The significance of each correlation was established using a percentile bootstrap method to compute confidence intervals for the correlation coefficient. Correlations between the two sites were also compared with a percentile bootstrap method. All statistical analyses were performed using the software R version 2.15.2 and the Wilcox Robust Statistics package (http://www.r-project.org).
Results
Water Analysis
There were no significant differences between the two sites for the average values of seawater temperature during the experimental season (p = 0.44) (ESM Appendix 2). The variables measured to characterize the water (i.e., total, organic, and inorganic particulates) varied significantly among experimental dates within each site. Nevertheless, these differences were relatively minor and likely not biologically relevant when compared to the major differences in seston observed between Milford Harbor and Hunts Point. Mean daily and mean of all measured values of TPM, POM, and PIM for each sampling site are shown in ESM Appendix 2. The organic content of the seston (f) was very different between the two locations because of high inorganic loads (PIM) at Hunts Point. Seston at Milford had a consistently higher percentage of organic content than Hunts Point (43.38 ± 0.97 vs. 22.58 ± 0.45 %, respectively).
Organic content (f) of the water as a function of the total particulate matter was plotted using all the measurements taken during the entire study period at each site (Fig. 1). The negative relationship between these two variables is presented in Table 2. There was a very strong negative relationship between total particulate matter and seston organic content at Hunts Point (r pb = −0.92), indicating that high total particulate matter in the water “diluted” the organic content. Conversely, at Milford, an increase in total particulate matter could be attributed either to increased inorganic or increased organic matter, resulting in a significantly lower correlation (r pb = 0.40; p < 0.001).
Mussel Characteristics
Mussel shell length, dry weight, GTT, and standard GTT were recorded and analyzed throughout the period of study (ESM Appendix 3). All subsequent statistical analyses and comparisons between sites are for mean and variance values from each site throughout the study. None of these variables showed significant differences between the sites (all p > 0.05).
Feeding Variables
Table 3 shows values of physiological measurements for a standard sized mussel (1 g dry mussel flesh) throughout the study period for each location. Site mean clearance rate was higher for mussels at Milford compared to Hunts Point (p < 0.001), but mean filtration rate was the same at both sites (p = 0.63). Thus, although ribbed mussels at Milford circulated more water through their gills, mussels from both sites filtered the same amount of particulate matter per unit of time. As a consequence, mussels from Hunts Point filtered much more inorganic matter, promoting active pre-ingestive selection, i.e., high production of pseudofeces. This is demonstrated by the site mean rejection proportion and selection efficiencies, both of which were higher for the Hunts Point mussels than Milford mussels (both p < 0.001). These results are corroborated by the relationship between the rejection proportion of the mussels and the total particulates in the water (Table 2). At Hunts Point, the correlation was significantly higher between these two variables (r pb = 0.64; p = 0.0033) than at Milford (r pb = 0.26). At Hunts Point, the more particulates present in the water, the more inorganic matter the water contained, resulting in a higher rejection proportion by the mussels. Moreover, the organic content of the ingested matter (i) for mussels at Hunts Point had a significantly higher correlation with the mussel rejection proportion than was observed for mussels at Milford Harbor (r pb = 0.71 vs. r pb = 0.27, respectively; p = 0.007) (Table 2), which is consistent with the observation that more total particulates resulted in more pseudofeces production, and thus, increased organic content of the ingested matter. Accordingly, the mean organic ingestion rate of mussels at Hunts Point was lower than at Milford (p < 0.001), and furthermore, the absorption rate was significantly lower at Hunts Point than at Milford Harbor (p < 0.001). The mean values of total ingestion rate, organic ingestion rate, and absorption rate were higher at Milford than Hunts Point (all p < 0.001), but mussels from both locations had the same mean absorption efficiency (p = 0.63). To visualize the feeding behavior of the mussels at each site, we plotted the relationships between clearance rate and total particulate matter for both sites (Fig. 2(a)). Clearance rate decreased significantly with total particulate matter in the water at both sites (Table 2). To compensate for the extremely high inorganic particle load, however, mussels at Hunts Point modulated GTT according to total particulate matter to permit longer exposure of ingested particles to digestive processes. This is apparent from the strong positive relationship between standard gut transit time and total particulate matter (Fig. 2(b); Table 2, r pb = 0.92, p = 0.01), i.e., longer gut transit times were observed when there were more total particulates. The same relationship was much weaker at Milford and the correlation was not significant (r pb = 0.30, p = 0.40; Table 2).
Discussion
Ribbed mussels from the two locations studied used different feeding strategies to adapt to the characteristics of the water they inhabited. The particulate loads measured at Milford Harbor and Hunts Point were above the limit of the pseudofeces threshold for mussels, which for M. edulis has been set at 5.0 mg L−1 (Widdows et al. 1979; Bayne et al. 1993). The differences in correlations observed between the organic content of the water (f) and the total particulate matter indicate two different types of seston. Hunts Point is representative of shallow, tidally mixed estuarine ecosystems; in these sites, tides, wind, and shallow depth result in resuspension of the sediment from the bottom, reducing the relative organic content of particles in the water column (Hawkins et al. 1996; Urrutia et al. 1996; Velasco and Navarro 2005; Galimany et al. 2011). Moreover, at Hunts Point, strong tidal currents and water inputs from the Bronx River favor the resuspension of material in the water column on a regular basis. For these reasons, high total particulate load in Hunts Point water resulted in relatively low organic content. Milford Harbor, in contrast, could experience high total particulates in the water without a high proportion of inorganic matter. This phenomenon has been observed in other estuarine ecosystems such as Great Sound (New Jersey, USA) (Fegley et al. 1992) and indicates the presence of phytoplankton and/or particulate organic matter that may serve as nutritious food for benthic, suspension feeders. The levels of particulate matter observed at Milford Harbor were similar to those reported by the CT Department of Energy and Environmental Protection during monthly monitoring off the coast of Milford from 2007 to 2009 (average 5.56 ± 0.46 mg L−1) (CT-DEEP 2011). Despite the relatively low seston organic content at Hunts Point, it appears possible, from studies elsewhere, that this site could not only support a small population of mussels but may also be able to support mussel aquaculture. For example, Gaeta Gulf (Sicily, Italy) is a mussel (Mytilus galloprovincialis) production site on the Mediterranean coast classified as “meso-trophic,” with mean total particulate matter of 4.8 ± 3.9 mg L−1 but low average organic content (0.22 mg L−1) (Mazzola et al. 1999; Sarà and Mazzola 2004).
It is appropriate to compare the feeding behavior of ribbed mussels to that of other mussel species because all mussels have the same fundamental gill structure. In our study, the common response of ribbed mussels from the two sites was to decrease clearance rate with increasing total particulates, similar to findings on M. galloprovincialis in a Mediterranean estuary (Galimany et al. 2011). On the other hand, Hawkins et al. (1996) found that clearance rates of blue mussels, M. edulis, increased as the total particulate load increased. This difference may be explained by the extremely high loads of particulate matter in the study of Hawkins et al. (1996) (10–90 mg mL−1), much higher than at Hunts Point. It seems likely that the blue mussels compensated for a low organic content in the seston by increasing clearance rate. The relationship between clearance rate and total particulates may well vary with species, physiological status, and food availability.
At Hunts Point, ribbed mussels employed several strategies to maximize nutritional gain from the water to which they were exposed. The mussels increased pseudofeces production in response to increased total particulates in the water. The organic content of the ingested matter was, therefore, enriched through pre-ingestive selection of organic particles. These results are consistent with strategies observed in mussels inhabiting other estuaries with relatively low organic content (Widdows et al. 1979; Bayne et al. 1993; Hawkins et al. 1996; Hawkins et al. 1998). Moreover, in this type of environment, other compensatory mechanisms for reduced dietary quality are possible, not only changes in feeding behavior but also in digestion and absorption (Bayne et al. 1988). It has been reported that longer retention of food in the digestive system of bivalves results in an increase in absorption efficiency; this relationship is thought to provide the bivalves with the physiological flexibility to compensate for reduced food quality (Bayne et al. 1984, 1988).
One concern about calculated values based upon GTT is our use of the minimum GTT observed in each experiment. We believe that this is unlikely to alter the findings across the series of measurements. The relationship between gut transit time and total particulate matter is very strong at Hunts Point. Moreover, it is likely that the individual ribbed mussel that produced green biodeposits the fastest was the least stressed by handling and, therefore, most representative of the mussels used for the feeding study.
The feeding behavior of bivalves also can be influenced by conditions other than food quantity and quality. For example, the amount of organic material ingested increases allometrically with the size of mussels (Navarro and Winter 1982; Pérez Camacho et al. 2000). In our study, the shell length of ribbed mussels was not significantly different between the two sites; thus, it is very unlikely that the feeding response differences were related to mussel size. Water temperature is also known to affect the physiological status of bivalves directly, regulating feeding behavior (Denis et al. 1999; MacDonald and Ward 2009). As an example, when temperature exceeds 25 °C, filtration rates fall significantly in M. galloprovincialis and in M. edulis (González and Yevich 1976; Anestis et al. 2007). Nevertheless, the differences found in the feeding behavior of the ribbed mussels between the two study sites were not likely related to temperature because the average values for water temperature at each location were not significantly different. Moreover, the temperatures at both locations were well within the tolerance range of G. demissa (below 0 to 36 °C) (Lent 1969).
The abilities and mechanisms of different bivalve species to adapt feeding processes to different environmental conditions vary widely (Hawkins et al. 1998). When selecting the best bivalve species to use for nutrient bioextraction purposes, endemic species are generally preferable because they are adapted to the local environment. It is worth noting that the present study did not quantify total nutrient removal by the mussels at Hunts Point; the present study was done in anticipation of a pilot-scale experiment. Future studies documenting rate and magnitude of removal of management-targeted nutrients such as nitrogen and phosphorus by ribbed mussels are recommended. The present study shows that ribbed mussels can adapt to a wide range of environmental conditions and find a feeding strategy to obtain the best yield given the available seston organic content. The findings of this study, combined with the known physiological plasticity of this bivalve, indicate that G. demissa is a good candidate species for use in cultivation-based nutrient reductions, particularly in highly impacted coastal ecosystems that cannot support traditional aquaculture of shellfish for human consumption.
References
Abbott, R.T. 1974. American seashells. New York: Litton.
Alber, M., and I. Valiela. 1994. Assimilation of organic aggregates by marine mussels. Marine Biology 121: 259–265.
Anestis, A., A. Lazou, H.O. Pörtner, and B. Michaelidis. 2007. Behavioral, metabolic, and molecular stress responses of marine bivalve Mytilus galloprovincialis during long-term acclimation at increasing ambient temperature. American Journal of Physiology—Regulatory, Integrative and Comparative Physiology 293: R911–R921.
Bayne, B.L., D.W. Klumpp, and K.R. Clarke. 1984. Aspects of feeding, including estimates of gut residence time, in three mytilid species (Bivalvia, Mollusca) at two contrasting sites in the Cape Peninsula, South Africa. Oecologia 64: 26–33.
Bayne, B.L., A.J.S. Hawkins, and E. Navarro. 1988. Feeding and digestion insuspension-feeding bivalve molluscs: the relevance of physiological compensation. American Zoologist 28: 147–159.
Bayne, B.L., J.I.P. Iglesias, A.J.S. Hawkins, E. Navarro, M. Heral, and J.M. Deslous Paoli. 1993. Feeding behaviour of the mussel, Mytilus edulis: responses to variations in quantity and organic content of the seston. Journal of the Marine Biological Association of the United Kingdom 73: 813–829.
Coen, L.D., R.D. Brumbaugh, D. Bushek, R. Grizzle, M.W. Luckenbach, M.H. Posey, S.P. Powers, and S.G. Tolley. 2007. Ecosystem services related to oyster restoration. Marine Ecology Progress Series 341: 303–307.
CT-DEEP. 2011. Long Island Sound Water Quality Monitoring Program Data 2007–2009. edited by C. Connecticut Department of Energy and Environmental Protection; Bureau of Water Protection and Land Reuse; Planning and Standards Division; Long Island Sound Water Quality Monitoring Program; Hartford.
Denis, L., E. Alliot, and D. Grzebyk. 1999. Clearance rate responses of Mediterranean mussels, Mytilus galloprovincialis, to variations in the flow, water temperature, food quality and quantity. Aquatic Living Resources 12: 279–288.
Doeller, J.E., M.K. Grieshaber, and D.W. Kraus. 2001. Chemolithoheterotrophy in a metazoan tissue: thiosulfate production matches ATP demand in ciliated mussel gills. The Journal of Experimental Biology 204: 3755–3764.
Fegley, S.R., B.A. MacDonald, and T.R. Jacobsen. 1992. Short-term variation in the quantity and quality of seston available to benthic suspension feeders. Estuarine, Coastal and Shelf Science 34: 393–412.
Galimany, E., M. Ramón, and I. Ibarrola. 2011. Feeding behavior of the mussel Mytilus galloprovincialis (L.) in a Mediterranean estuary: a field study. Aquaculture 314: 236–243.
Galimany, E., J.H. Alix, M.S. Dixon, and G.H. Wikfors. 2012. Short communication: adaptability of the feeding behavior of intertidal ribbed mussels (Geukensia demissa) to constant submersion. Aquaculture International. doi:10.1007/s10499-012-9608-3.
González, G.J., and P. Yevich. 1976. Responses of an estuarine population of the blue mussel Mytilus edulis to heated water from a steam generating plant. Marine Biology 34: 177–189.
Hart, R. 2003. Dynamic pollution control-time lags and optimal restoration of marine ecosystems. Ecological Economics 47: 79–93.
Hawkins, A.J.S., E. Navarro, and J.I.P. Iglesias. 1990. Comparative allometries of gut-passage time, gut content and metabolic faecal loss in Mytilus edulis and Cerastoderma edule. Marine Biology 105: 197–204.
Hawkins, A.J.S., R.F.M. Smith, B.L. Bayne, and M. Héral. 1996. Novel observations underlying the fast growth of suspension-feeding shellfish in turbid environments: Mytilus edulis. Marine Ecology Progress Series 131: 179–190.
Hawkins, A.J.S., B.L. Bayne, S. Bougrier, M. Héral, J.I.P. Iglesias, E. Navarro, R.F.M. Smith, and M.B. Urrutia. 1998. Some general relationships in comparing the feeding physiology of suspension-feeding bivalve molluscs. Journal of Experimental Marine Biology and Ecology 219: 87–103.
Heck Jr., K.L., and J.F. Valentine. 2007. The primacy of top-down effects in shallow benthic ecosystems. Estuaries and Coasts 30: 371–381.
Iglesias, J.I.P., M.B. Urrutia, E. Navarro, and I. Ibarrola. 1998. Measuring feeding and absorption in suspension-feeding bivalves: an appraisal of the biodeposition method. Journal of Experimental Marine Biology and Ecology 219: 71–86.
Jordan, T.E., and I. Valiela. 1982. A nitrogen budget of the ribbed mussel, Geukensia demissa, and its significance in nitrogen flow in a New England salt marsh. Limnology and Oceanography 27: 75–90.
Kemp, W.M., W.R. Boynton, J.E. Adolf, D.F. Boesch, W.C. Boicourt, G. Brush, J.C. Cornwell, T.R. Fisher, P.M. Glibert, J.D. Hagy, L.W. Harding, E.D. Houde, D.G. Kimmel, W.D. Miller, R.I.E. Newell, M.R. Roman, E.M. Smith, and J.C. Stevenson. 2005. Eutrophication of Chesapeake Bay: historical trends and ecological interactions. Marine Ecology Progress Series 303: 1–29.
Kreeger, D.A., and R.I.E. Newell. 1996a. Ingestion and assimilation of carbon from cellulolytic bacteria and heterotrophic flagellates by the mussels Geukensia demissa and Mytilus edulis (Bivalvia, Mollusca). Aquatic Microbial Ecology 11: 205–214.
Kreeger, D.A., and R.I.E. Newell. 1996b. Omnivory by the mussel, Geukensia demissa. Journal of Shellfish Research 15: 506–507.
Kreeger, D.A., and R.I.E. Newell. 2001. Seasonal utilization of different seston carbon sources by the ribbed mussel, Geukensia demissa (Dillwyn) in a mid-Atlantic salt marsh. Journal of Experimental Marine Biology and Ecology 260: 71–91.
Kreeger, D., P. Cole, D. Bushek, K. Kraueter, and J. Adkins. 2011. Marine bivalve shellfish conservation priorities for the Delaware Estuary. Partnership for the Delaware Estuary (PDE) PDE Report #11-03: 54.
Langdon, C.J., and R.I.E. Newell. 1990. Utilization of detritus and bacteria as food sources by 2 bivalve suspension-feeders, the oyster Crassostrea virginica and the mussel Geukensia demissa. Marine Ecology Progress Series 58: 299–310.
Lee, R.W., D.W. Kraus, and J.E. Doeller. 1996. Sulfide-stimulation of oxygen consumption rate and cytochrome reduction in gills of the estuarine mussel Geukensia demissa. The Biological Bulletin 191: 421–430.
Lent, C.M. 1969. Adaptations of the ribbed mussel, Modiolus demissus (Dillwyn), to the intertidal habitat. American Zoologist 9: 283–294.
Lindahl, O. 2011. Mussel farming as a tool for re-eutrophication of coastal waters: experiences from Sweden. In Shellfish aquaculture and the environment, ed. S.E. Shumway, 217–237. Chichester: Wiley.
Lindahl, O., and S. Kollberg. 2009. Can the EU agri-environmental aid program be extended into the coastal zone to combat eutrophication? Hydrobiologia 629: 59–64.
MacDonald, B.A., and J.E. Ward. 2009. Feeding activity of scallops and mussels measured simultaneously in the field: repeated measures sampling and implications for modelling. Journal of Experimental Marine Biology and Ecology 371: 42–50.
Manganaro, A., G. Pulicanò, A. Reale, M. Sanfilippo, and G. Sarà. 2009. Filtration pressure by bivalves affects the trophic conditions in Mediterranean shallow ecosystems. Chemistry and Ecology 25: 467–478.
Mazzola, A., T.L. Rosa, B. Savona, and G. Sarà. 1999. Seston dynamics and food availability in a mussel system (Gulf of Gaeta, southern Tyrrhenian Sea, Italy). Journal of Shellfish Research 18: 331.
Navarro, J.M., and J.E. Winter. 1982. Ingestion rate, assimilation efficiency and energy balance in Mytilus chilensis in relation to body size and different algal concentration. Marine Biology 67: 255–266.
Nixon, S.W. 1995. Coastal marine eutrophication: a definition, social causes, and future concerns. Ophelia 41: 199–219.
Ostroumov, S.A. 2005. Some aspects of water filtering activity of filter-feeders. Hydrobiologia 542: 275–286.
Pérez Camacho, A., U. Labarta, and E. Navarro. 2000. Energy balance of mussels Mytilus galloprovincialis: the effect of length and age. Marine Ecology Progress Series 199: 149–158.
Peterson, B.J., R.W. Howarth, and R.H. Garritt. 1985. Multiple stable isotopes used to trace the flow of organic matter in estuarine food webs. Science 227: 1361–1363.
Riisgård, H.U. 1988. Efficiency of particle retention and filtration rate in 6 species of Northeast American bivalves. Marine Ecology Progress Series 45: 217–223.
Rose, J.M., M. Tedesco, G.H. Wikfors, and C. Yarish. 2010. International Workshop on Bioextractive Technologies for Nutrient Remediation Summary Report. N.F.S.C.R. US Dept Commer: 12.
Sarà, G., and A. Mazzola. 2004. The carrying capacity for Mediterranean bivalve suspension feeders: evidence from analysis of food availability and hydrodynamics and their integration into a local model. Ecological Modelling 179: 281–296.
Stephenson, K., S. Aultman, T. Metcalfe, and A. Miller. 2010. An evaluation of nutrient nonpoint offset trading in Virginia: a role for agricultural nonpoint sources? Water Resources Research 46, W04519. doi:10.1029/2009WR008228.
Urrutia, M.B., J.I.P. Iglesias, E. Navarro, and J. Prou. 1996. Feeding and absorption in Cerastoderma edule under environmental conditions in the bay of Marennes Oleron (Western France). Journal of Experimental Marine Biology and Ecology 76: 431–450.
Velasco, L.A., and J.M. Navarro. 2005. Feeding physiology of two bivalves under laboratory and field conditions in response to variable food concentrations. Marine Ecology Progress Series 291: 115–124.
Widdows, J., P. Fieth, and C.M. Worrall. 1979. Relationships between seston, available food and feeding activity in the common mussel Mytilus edulis. Marine Biology 50: 195–207.
Wilcox, R.R. 2003. Applying contemporary statistical techniques. New York: Academic.
Wright, R.T., R.B. Coffin, C.P. Ersing, and D. Pearson. 1982. Field and laboratory measurements of bivalve filtration of natural marine bacteroplankton. Limnology and Oceanography 27: 91–98.
Acknowledgments
This work was partially supported by a Research Associateship to the first author from the National Research Council with funding from Dr. Michael Rubino of the NOAA National Aquaculture Program. We would like to thank Robert Alix and Werner Schreiner for their indispensable help fabricating and modifying field sampling equipment. We would like to acknowledge Daphne Belfodil for her technical support in the field and laboratory. We also want to acknowledge Brian Giordano for allowing us to work at the Hunts Point site and being always so helpful. Last but not the least, we want to thank the staff and apprentices from Rocking the Boat.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Appendix 1
(DOC 318 kb)
ESM Appendix 2
(DOC 45 kb)
ESM Appendix 3
(DOC 48 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Galimany, E., Rose, J.M., Dixon, M.S. et al. Quantifying Feeding Behavior of Ribbed Mussels (Geukensia demissa) in Two Urban Sites (Long Island Sound, USA) with Different Seston Characteristics. Estuaries and Coasts 36, 1265–1273 (2013). https://doi.org/10.1007/s12237-013-9633-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-013-9633-0