Abstract
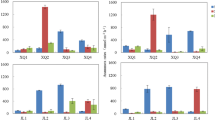
Anammox bacteria are widespread in the marine environment, but studies of anammox in marshes and other wetlands are still scarce. In this study, the role of anammox in nitrogen removal from marsh sediments was surveyed in four vegetation types characteristic of New England marshes and in unvegetated tidal creeks. The sites spanned a salinity gradient from 0 to 20 psu. The impact of nitrogen loading on the role of anammox in marsh sediments was studied in a marsh fertilization experiment and in marshes with high nitrogen loading entering through ground water. In all locations, nitrogen removal through anammox was low compared to denitrification, with anammox accounting for less than 3% of the total N2 production. The highest relative importance of anammox was found in the sediments of freshwater-dominated marshes, where anammox approached 3%, whereas anammox was of lesser importance in saline marsh sediments. Increased nitrogen loading, in the form of nitrate from natural or artificial sources, did not impact the relative importance of anammox, which remained low in all the nitrogen enriched locations (<1%).





Similar content being viewed by others
References
Bodelier, P.L.E., J.A. Libochant, C. Blom, and H.J. Laanbroek. 1996. Dynamics of nitrification and denitrification in root-oxygenated sediments and adaptation of ammonia-oxidizing bacteria to low-oxygen or anoxic habitats. Applied and Environmental Microbiology 62: 4100–4107.
Boran, K., M. Kolevab, R. Arsovb, W. van der Starc, M.S.M. Jetten, and M. Strous. 2006. Adaptation of a freshwater anammox population to high salinity wastewater. Journal of Biotechnology 126: 546–553. doi:10.1016/j.jbiotec.2006.05.012.
Brandes, J.A., A.H. Devol, and C. Deutsch. 2007. New developments in the marine nitrogen cycle. Chemical Reviews 107: 577–589. doi:10.1021/cr050377t.
Burgin, A., and S. Hamilton. 2007. Nitrate removal in aquatic ecosystems. Frontiers in Ecology and the Environment 5: 89–96. doi:10.1890/1540-9295(2007)5[89:HWOTRO]2.0.CO;2.
Crompton, T.R. 2005. Analysis of seawater—a guide for the analytical and environmental chemist. Berlin: Springer.
Dalsgaard, T., D.E. Canfield, J. Petersen, B. Thamdrup, and J. Acuna-Gonzalez. 2003. N-2 production by the anammox reaction in the anoxic water column of Golfo Dulce, Costa Rica. Nature 422: 606–608. doi:10.1038/nature01526.
Dalsgaard, T., B. Thamdrup, and D.E. Canfield. 2005. Anaerobic ammonium oxidation (anammox) in the marine environment. Research in Microbiology 156: 457–464. doi:10.1016/j.resmic.2005.01.011.
Deegan, L.A., J.L. Bowen, D. Drake, J.W. Fleeger, C.T. Friedrichs, K.A. Galvan, J.E. Hobble et al. 2007. Susceptibility of salt marshes to nutrient enrichment and predator removal. Ecological Applications 17: S42–S63. doi:10.1890/06-0452.1.
Dollhopf, S.L., J.H. Hyun, A.C. Smith, H.J. Adams, S. O’Brien, and J.E. Kostka. 2005. Quantification of ammonia-oxidizing bacteria and factors controlling nitrification in salt marsh sediments. Applied and Environmental Microbiology 71: 240–246. doi:10.1128/AEM.71.1.240-246.2005.
Dong, Z.Q., and T.H. Sun. 2007. A potential new process for improving nitrogen removal in constructed wetlands—promoting coexistence of partial-nitrification and ANAMMOX. Ecological Engineering 31: 69–78. doi:10.1016/j.ecoleng.2007.04.009.
Engstrom, P., T. Dalsgaard, S. Hulth, and R.C. Aller. 2005. Anaerobic ammonium oxidation by nitrite (anammox): Implications for N-2 production in coastal marine sediments. Geochimica Et Cosmochimica Acta 69: 2057–2065. doi:10.1016/j.gca.2004.09.032.
Francis, C.A., J.M. Beman, and M.M.M. Kuypers. 2007. New processes and players in the nitrogen cycle: the microbial ecology of anaerobic and archaeal ammonia oxidation. Isme Journal 1: 19–27. doi:10.1038/ismej.2007.8.
Hamersley, M.R. 2002. The role of denitrification in the nitrogen cycle of new england salt marshes. Ph.D. thesis, Woods Hole Oceanographic Institution/Massachusetts Institute of Technology, Woods Hole, MA, USA.
Hammersley, M.R., G. Lavik, D. Woebken, J.E. Rattray, P. Lam, H. Hopmans, J.S. Sinninghe Damsté et al. 2007. Ammonium oxidation contributes significantly to nitrogen loss from the Peruvian oxygen minimum zone. Limnology and Oceanography 52: 923–933.
Hopkinson, C., and A. Giblin. 2008. Nitrogen dynamics in salt marsh ecosystems. In Nitrogen in the marine environment, eds. D. Capone, D. Bronk, D. Mulholland, and E. CarpenterNew York: Academic.
Kana, T.M., C. Darkangelo, M.D. Hunt, J.B. Oldham, G.E. Bennett, and J.C. Cornwell. 1994. Membrane inlet mass-spectrometer for rapid high-precision determination of N-2, O-2, and Ar in Environmental Water Samples. Analytical Chemistry 66: 4166–4170. doi:10.1021/ac00095a009.
Kuypers, M.M.M., G. Lavik, D. Woebken, M. Schmid, B.M. Fuchs, R. Amann, B.B. Jorgensen et al. 2005. Massive nitrogen loss from the Benguela upwelling system through anaerobic ammonium oxidation. Proceedings of the National Academy of Sciences of the United States of America 102: 6478–6483. doi:10.1073/pnas.0502088102.
Magalhaes, C.M., S.B. Joye, R.M. Moreira, W.J. Wiebe, and A.A. Borlado. 2005. Effect of salinity and inorganic nitrogen concentrations on nitrification and denitrification rates in intertidal sediments and rocky biofilms of the Douro River estuary, Portugal. Water Research 39: 1783–1794. doi:10.1016/j.watres.2005.03.008.
Matheson, F.E., M.L. Nguyen, A.B. Cooper, and T.P. Burt. 2003. Short-term nitrogen transformation rates in riparian wetland soil determined with nitrogen-15. Biology and Fertility of Soils 38: 129–136. doi:10.1007/s00374-003-0640-3.
Meyer, R.L., N. Risgaard-Petersen, and D.E. Allen. 2005. Correlation between anammox activity and microscale distribution of nitrite in a subtropical mangrove sediment. Applied and Environmental Microbiology 71: 6142–6149. doi:10.1128/AEM.71.10.6142-6149.2005.
Mulder, A., A.A. Vandegraaf, L.A. Robertson, and J.G. Kuenen. 1995. Anaerobic ammonium oxidation discovered in a denitrifying fluidized-bed reactor. FEMS Microbiology Ecology 16: 177–183. doi:10.1111/j.1574-6941.1995.tb00281.x.
Paredes, D., P. Kuschk, and H. Koser. 2007. Influence of plants and organic matter on the nitrogen removal in laboratory-scale model subsurface flow constructed wetlands inoculated with anaerobic ammonium oxidizing bacteria. Engineering in Life Sciences 7: 565–576. doi:10.1002/elsc.200700030.
Penton, C.R., A.H. Devol, and J.M. Tiedje. 2006. Molecular evidence for the broad distribution of anaerobic ammonium-oxidizing bacteria in freshwater and marine sediments. Applied and Environmental Microbiology 72: 6829–6832. doi:10.1128/AEM.01254-06.
Reddy, K.R., W.H. Patrick, and C.W. Lindau. 1989. Nitrification–denitrification at the plant root–sediment interface in wetlands. Limnology and Oceanography 34: 1004–1013.
Rich, J.J., O.R. Dale, B. Song, and B.B. Ward. 2008. Anaerobic ammonium oxidation (Anammox) in Chesapeake Bay sediments. Microbial Ecology 55: 311–320. doi:10.1007/s00248-007-9277-3.
Risgaard-Petersen, N., R.L. Meyer, M. Schmid, M.S.M. Jetten, A. Enrich-Prast, S. Rysgaard, and N.P. Revsbech. 2004. Anaerobic ammonium oxidation in an estuarine sediment. Aquatic Microbial Ecology 36: 293–304. doi:10.3354/ame036293.
Schmid, M.C., N. Risgaard-Petersen, J. van de Vossenberg, M.M.M. Kuypers, G. Lavik, J. Petersen, S. Hulth et al. 2007. Anaerobic ammonium-oxidizing bacteria in marine environments: widespread occurrence but low diversity. Environmental Microbiology 9: 1476–1484. doi:10.1111/j.1462-2920.2007.01266.x.
Seitzinger, S.P. 1988. Denitrification in freshwater and coastal marine ecosystems: ecological and geochemical significance. Limnology and Oceanography 33: 702–724.
Seitzinger, S.P., W.S. Gardner, and A.K. Spratt. 1991. The effect of salinity on ammonium sorption in aquatic sediments: Implications for benthic nutrient recycling. Estuaries 14: 167–174. doi:10.2307/1351690.
Sherr, B.F., and W.J. Payne. 1978. Effect of the Spartina alterniflora root–rhizome system on salt marsh soil denitrifying bacteria. Applied and Environmental Microbiology 35: 724–729.
Strous, M., J.A. Fuerst, E.H.M. Kramer, S. Logemann, G. Muyzer, K.T. van de Pas-Schoonen, R. Webb et al. 1999. Missing lithotroph identified as new planctomycete. Nature 400: 446–449. doi:10.1038/22749.
Teal, J.M., and B.L. Howes. 2000. Salt marsh values: Retrospection from the end of the century. In Concepts and controversies in tidal marsh ecology, eds. M.P. Weinstein, and D.A. Kreeger, 9–19. Dordrecht: Kluwer Academic.
Thamdrup, B., and T. Dalsgaard. 2002. Production of N-2 through anaerobic ammonium oxidation coupled to nitrate reduction in marine sediments. Applied and Environmental Microbiology 68: 1312–1318. doi:10.1128/AEM.68.3.1312-1318.2002.
Thompson, S.P., H.W. Paerl, and M.C. Go. 1995. Seasonal patterns of nitrification and denitrification in a natural and a restored salt-marsh. Estuaries 18: 399–408. doi:10.2307/1352322.
Trimmer, M., J.C. Nicholls, and B. Deflandre. 2003. Anaerobic ammonium oxidation measured in sediments along the Thames estuary, United Kingdom. Applied and Environmental Microbiology 69: 6447–6454. doi:10.1128/AEM.69.11.6447-6454.2003.
Valiela, I., and M.L. Cole. 2002. Comparative evidence that salt marshes and mangroves may protect seagrass meadows from land-derived nitrogen loads. Ecosystems 5: 92–102. doi:10.1007/s10021-001-0058-4.
Van de Graaf, A.A., A. Mulder, P. Debruijn, M.S.M. Jetten, L.A. Robertson, and J.G. Kuenen. 1995. Anaerobic oxidation of ammonium is a biologically mediated process. Applied and Environmental Microbiology 61: 1246–1251.
Van de Graaf, A.A., P. deBruijn, L.A. Robertson, M.S.M. Jetten, and J.G. Kuenen. 1996. Autotrophic growth of anaerobic ammonium-oxidizing micro-organisms in a fluidized bed reactor. Microbiology 142: 2187–2196.
van de Vossenberg, J., J.E. Rattray, W. Geerts, B. Kartal, L. van Niftrik, E. van Donselaar, J.S. Sinninghe Damste et al. 2008. Enrichment and characterization of marine anammox bacteria associated with global nitrogen gas production. Functional diversity of fresh water and marine anammox bacteria. Environmental Microbiology 10: 3120–3129. doi:10.1111/j.1462-2920.2008.01643.x.
Acknowledgments
The research was supported by the National Science Foundation PIE-LTER (NSF-OCE-9726921; NSF-OCE-0423565); NSF DEB 0213767 and the NOAA, Department of Commerce under Grant number NA16RG2273, Woods Hole Oceanographic Institutions Sea Grant project R/M-50 and R/M-53. The views expressed here are those of the authors and so do not necessarily reflect the views of NOAA or any of its sub-agencies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koop-Jakobsen, K., Giblin, A.E. Anammox in Tidal Marsh Sediments: The Role of Salinity, Nitrogen Loading, and Marsh Vegetation. Estuaries and Coasts 32, 238–245 (2009). https://doi.org/10.1007/s12237-008-9131-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-008-9131-y