Abstract
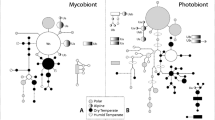
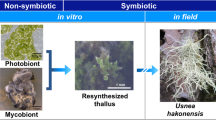
It is proposed that lichen photobionts, compared to mycobionts, have very limited capacity to evolve adaptations to lichenization, so that the symbionts in lichens do not co-evolve. This is because lichens have (a) no sequential selection of photobiont cells from one lichen into another needed for Darwinian natural selection and (b) no photobiont sexual reproduction in the thallus. Molecular studies of lichen photobionts indicate no predictable patterns of photobiont lineages that occur in lichens so supporting this proposal. Any adaptation by photobionts accumulating beneficial mutations for lichenization is probably insignificant compared to the rate of mycobiont adaptation. This proposal poses questions for research relating the photobiont sexual cycle (genetic and cellular), the fate of photobiont lineages after lichenization, whether lineages of photobionts in thalli change with time, thallus formation by from spores as well as carbohydrate movement from photobionts to mycobionts and regulation of co-development of the symbionts in the thallus.
Similar content being viewed by others
Literature Cited
Ahmadjian, V. 1993a. The Lichen Symbiosis (2nd ed.). Wiley, New York.
———. 1993b. The lichen photobiont—what can it tell us about lichen systematics. Bryologist 96: 310–313
Aproot, A. & C. M. van Herk. 2007. Further evidence of the effects of global warming on lichens, particularly those with Trentepohlia phycobionts. Environmental Pollution 146: 293–298.
Baloch, E. & M. Grube. 2006. Evolution and phylogenetic relationships within Porinaceae (Ostropomycetidae), focusing on foliicolous species. Mycological Research 110: 125–136.
Barraclough, T. G., D. Fontaneto, C. Ricci & E. A. Herniou. 2007. Evidence for inefficient selection against deleterious mutations in cytochrome oxidase I of asexual bdelloid rotifers. Molecular and Biological Evolution 24: 1952–1962.
Beck, A. & H. U. Koop. 2001. Analysis of the photobiont population in lichens using a single-cell manipulator. Symbiosis 31: 57–67.
———, T. Friedl & G. Rambold. 1998. Selectivity of photobiont choice in a defined lichen community: inferences from cultural and molecular studies New Phytologist 139: 709–720.
———, T. Kasalicky & G. Rambold. 2002. Myco-photobiontal selection in a Mediterranean cryptogam community with Fulgensia fulgida. New Phytologist 153: 317–326.
Beiggi, S. & M. D. Piercey-Normore. (2007) Evolution of ITS ribosomal RNA secondary structures in fungal and algal symbionts of selected species of Cladonia sect. Cladonia (Cladoniaceae, Ascomycotina). Journal of Molecular Evolution 64: 528–542.
Berg, C. C. & J. T. Wiebes. 1992. African fig trees and fig wasps. Koninklijke Nederlandse Akademie van Wetenschappen, Amsterdam.
Birky, C. W., C. Wolf, H. Maughan, L. Herbertson & E. Henry. 2005. Speciation and selection without sex. Hydrobiologia 546: 29–45.
Blaha, J., E. Baloch & M. Grube. 2006. High photobiont diversity associated with the euryoecious lichen-forming ascomycete Lecanora rupicola (Lecanoraceae, Ascomycota). Biological Journal of the Linnean Society 88: 283–293.
Cairney, J. W. G. 2000. Evolution of mycorrhiza systems. Naturwissenschaften 87: 467–475.
Castagnone-Sereno P. 2006. Genetic variability and adaptive evolution inparthenogenetic root-knot nematodes. Heredity 96: 282–289.
Cordeiro, L. M. C., R. A. Reis, L. M. Cruz, E. Stocker-Worgotter, M. Grube & M. Iacomini. 2005. Molecular studies of photobionts of selected lichens from the coastal vegetation of Brazil. FEMS Microbiology Ecology 54: 381–390.
de Meeûs, T., F. Prugnolle & P. Agnew. 2007. Asexual reproduction: genetics and evolutionary aspects. Cellular and Molecular Life Science 64: 1355–1372
DePriest, P. T. 2004. Early molecular investigations of lichen-forming symbionts:1986–2001. Annual Review of Microbiology 58: 273–301.
Ellis, C. J. & B. J. Coppins. 2007. Reproductive traits indicate successional dynamics of crustose lichen communities on aspen (Populus tremula L.) in Scotland. The Lichenologist 39: 377–391.
Fontaneto, D., E. A. Herniou, C. Boschetti, M. Caprioli, G. Melone, C. Ricci & T.G. Barraclough 2007. Independently evolving species in asexual bdelloid rotifers. PLoS Biology 5: 914–921 (www.plosbiology.org)
Friedl, T. & B. Büdel (1996) Photobionts. Pp. 8–23. In: Nash, T.H. (ed.), Lichen Biology. Cambridge University Press, Cambridge.
Fröberg, L., L. O. Bjorn, A. Baur & B. Baur 2001. Viability of lichen photobionts after passing through the digestive tract of a land snail. Lichenologist 33: 543–545.
Guzow-Krzeminska, B. 2006. Photobiont flexibility in the lichen Protoparmeliopsis muralis as revealed by ITS rDNA analyses. Lichenologist 38: 469–476.
Hawksworth, D. L. 1987. Observations on three algicolous microfungi. Notes of the Royal Botanic Garden, Edinburgh 44: 549–560
———. 1988. The variety of fungal-algal symbioses, their evolutionary significance and the nature of lichens. Botanical Journal of the Linnean Society 96: 3–20
——— & D. J. Hill. 1984. The Lichen-Forming Fungi. Blackie, Glasgow.
Hedenås, H., P. Blomberg & L. Ericson. 2007. Significance of old aspen (Populus tremula) trees for the occurrence of lichen photobionts. Biological Conservation 135: 380–387.
Helms G, T. Friedl, G. Rambold & H. Mayrhofer. 2001. Identification of photobionts from the lichen family Physciaceae using algal-specific ITS rDNA sequencing. Lichenologist 33: 73–86.
Hill D. J. 1976. The physiology of the lichen symbiosis. Pp. 457–496. In: Brown D. H., Hawksworth D. L. & Bailey R. H. (eds.), Lichenology: Progress and Problems. Academic, London.
———. 1992a. An overlooked symbiosis. Photosynthesis Research 34: 339–340.
———. 1992b. The co-ordination of development of symbionts in mutualistic symbiosis with reference to the cell cycle of the photobiont in lichens. Symbiosis 14: 325–333.
———. 1994a. The cell cycle of the photobiont of the lichen Parmelia sulcata (Lecanorales, Ascomycotina) during the development of thallus lobes. Cryptogamic Botany 4 270–273.
———. 1994b. The succession of lichens on gravestones: a preliminary investigation. Cryptogamic Botany 4: 179–186.
Honegger, R. 1993. The developmental biology of lichens. New Phytologist 125: 659–677
Hyvärinen, M., R. Hardling & J. Tuomi. 2002. Cyanobacterial lichen symbiosis: the fungal partner as an optimal harvester. Oikos 98: 498–504.
Kappen, L. 1994. The lichen, a mutualistic system—some mainly ecophysiological aspects. Cryptogamic Botany 4: 193–202.
Kuhn, G., M. Hijri & I. R. Sanders. 2001. Evidence for the evolution of multiple genomes in arbuscular mycorrhizal fungi. Nature 414: 745–748.
Law, R. & D. H. Lewis. 1983. Biotic environment and the maintenance of sex—some evidence from mutualisic symbiosis. Biological Journal of the Linnean Society 20: 249–276.
Lohtander, K., I. Oksanen & J. Rikkinen. 2003. Genetic diversity of green algal and cyanobacterial photobionts in Nephroma (Peltigerales). Lichenologist 35: 325–339.
Meeks, J. C. & J. Elhai. 2002. Regulation of cellular differentiation in filamentous cyanobacteria in free-living and plant-associated symbiotic growth states. Microbiology and Molecular Biology Reviews 66: 94–121.
Meier, F. A., S. Scherrer & R. Honegger. 2002. Faecal pellets of lichenivorous mites contain viable cells of the lichen-forming ascomycete Xanthoria parietina and its green algal photobiont, Trebouxia arboricola. Biological Journal of the Linnean Society 76: 259–268.
Muggia, L., M. Grube & M. Tretiach. 2008. A combined molecular and morphological approach to species delimitation in black-fruited, endolithic Caloplaca: high genetic and low morphological diversity. Mycological Research 112: 36–49.
Nelsen, M. P., & A. Gargas. 2008. Dissociation and horizontal transmission of codispersing lichen symbionts in the genus Lepraria (Lecanorales: Stereocaulaceae) New Phytologist 177: 264–275.
O’Brien, H. E., J. Miadlikowska & F. Lutzoni. 2005. Assessing host specialization in symbiotic cyanobacteria associated with four closely related species of the lichen fungus Peltigera. European Journal of Phycology 40: 363–378.
Ohmura, Y., M. Kawachi, F. Kasai, M. M. Watanabe & S. Takeshita. 2006. Genetic combinations of symbionts in a vegetatively reproducing lichen, Parmotrema tinctorum, based on ITS rDNA sequences. Bryologist 109: 43–59.
Palmqvist, K. 2000. Carbon economy in lichens. New Phytologist 148: 11–36.
Piercey-Normore, M. D. 2006. The lichen-forming ascomycete Evernia mesomorpha associates with multiple genotypes of Trebouxia jamesii. New Phytologist 169: 331–344.
——— & P. T. DePriest. 2001. Algal switching among lichen symbioses. American Journal of Botany 88: 1490–1498.
Proctor, M., P. Yeo & A. Lack. 1996. The Natural History of Pollination. HarperCollins, London.
Rikkinen, J. 2002. Cyanobionts evolutionary overview. Pp.31–72. In: Rae, A. N. (ed.), Cyanobacteria in Symbiosis. Dordrecht: Kluwer.
———. 2003. Ecological and evolutionary role of photobiont-mediated guilds in lichens. Symbiosis 34: 99–110.
Sanders, W. B. 2001. Composite lichen thalli of Sticta sp. from Brazil, with morphologically similar lobes containing either a chlorobiont or a cyanobiont layer. Symbiosis 31: 47–55.
———, R. L. Moe & C. Ascaso. 2005. Ultrastructural study of the brown alga Petroderma maculiforme (Phaeophyceae) in the free-living state and in lichen symbiosis with the intertidal marine fungus Verrucaria tavaresiae (Ascomycotina). European Journal of Phycology 40: 353–361.
Sipman, H. J. M. & A. Aproot. 2001. Where are the missing lichens? Mycological Research 105: 1433–1439.
Slocum, R. D., V. Ahmadjian & K. C. Hildreth. 1980. Zoosporogenesis in Trebouxia-gelatinosa - ultrastructure potential for zoospore release and implications for the lichen association. Lichenologist 12: 173–187.
Smith, D. C. 1980. Mechanisms of nutrient movement between the lichen symbionts. Pp. 197–227. In: Cook. C. L., Pappas, P. W. & Rudolph, E. D. (eds.), Cellular Interactions in Symbiosis and Parasitism. Ohio State University Press, Columbus.
Summerfield, T. C., D. J. Galloway & J. J. Eaton-Rye. 2002. Species of cyanolichens from Pseudocyphellaria with indistinguishable ITS sequences have different photobionts. New Phytologist 155: 121–129.
Tibell, L. 2001. Photobiont association and molecular phylogeny of the lichen genus Chaenotheca. Bryologist 104: 191–198.
Van Sandt, V. S. T., H. Stieperaere, Y. Guisez, J.-P. Verbelen & K. Vissenberg. 2007. XET activity is found near sites of growth and cell elongation in bryophytes and some green algae: new insights into the evolution of primary cell wall elongation. Annals of Botany 99: 39–51.
Wedin, M., H. Doring, K. Konberg & G. Gilenstam. 2005. Generic delimitations in the family Stictidaceae (Ostropales, Ascomycota): the Stictis-Conotrema problem. Lichenologist 37: 67–75.
Yahr, R., R. Vilgalys & P. T. Depriest. 2004. Strong fungal specificity and selectivity for algal symbionts in Florida scrub Cladonia lichens. Molecular Ecology 13: 3367–3378.
Zoller, S. & F. Lutzoni. 2003. Slow algae, fast fungi: exceptionally high nucleotide substitution rate differences between lichenized fungi Omphalina and their symbiotic green algae Coccomyxa. Molecular Phylogenetics and Evolution 29: 629–640.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hill, D.J. Asymmetric Co-evolution in the Lichen Symbiosis Caused by a Limited Capacity for Adaptation in the Photobiont. Bot. Rev. 75, 326–338 (2009). https://doi.org/10.1007/s12229-009-9028-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12229-009-9028-x