Abstract
Lower respiratory tract infection due to Pseudomonas aeruginosa has become increasingly challenging, resulting in a worse morbidity and mortality. Airway remodeling is a common phenomenon in this process, to which epithelial-mesenchymal transition (EMT) may contribute as an important promoter. Previous studies showed that epithelium-specific integrin αvβ6–mediated EMT was involved in pulmonary fibrosis via transforming growth factor-β1 (TGF-β1) signaling, but whether integrin αvβ6 plays a role in the P. aeruginosa–associated airway remodeling remains unknown. BEAS-2B cells were incubated with lipopolysaccharide (LPS) from P. aeruginosa in the presence or the absence of integrin αvβ6–blocking antibodies. Morphologic changes were observed by an inverted microscopy. The EMT markers were detected using Western blotting and immunofluorescence. The activation of TGF-β1-Smad2/3 signaling pathway was assessed. Furthermore, matrix metalloproteinase (MMP)-2 and -9 in the medium were measured using ELISA. P. aeruginosa’s LPS decreased the expression of the epithelial marker E-cadherin and promoted the mesenchymal markers, vimentin and α-smooth muscle actin in BEAS-2B cells. The expression of integrin αvβ6 was significantly increased during EMT process. Blocking integrin αvβ6 could attenuate P. aeruginosa’s LPS-induced EMT markers’ expression via TGF-β1-Smad2/3 signaling pathway. Furthermore, blocking integrin αvβ6 could prevent morphologic changes and oversecretion of MMP-2 and -9. Integrin αvβ6 mediates epithelial-mesenchymal transition in human bronchial epithelial cells induced by lipopolysaccharides of P. aeruginosa via TGF-β1-Smad2/3 signaling pathway and might be a promising therapeutic target for P. aeruginosa–associated airway remodeling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pseudomonas aeruginosa is one of the most frequent opportunistic pathogens, responsible for serious lower respiratory tract infection in patients with cystic fibrosis (Moore and Mastoridis 2017), chronic obstructive pulmonary disease (Almagro et al. 2012), bronchiectasis (Sanchez-Munoz et al. 2016), and accounting for 17.8% of all isolates in hospital-acquired pneumonia (Ding et al. 2016). Due to severe drug resistance and limited therapeutic options, P. aeruginosa–caused lower respiratory tract infection is becoming difficult to treat, which often results in high mortality in these patients (Zhuo et al. 2008). Emerging evidence have revealed that P. aeruginosa is associated with airway remodeling (Botha et al. 2008; Vos et al. 2008; Cigana et al. 2016), which is characterized by aberrant repair of the epithelium and accumulation of fibroblasts and could give rise to irreversible decline of pulmonary function and poor prognosis. However, little is known how to alleviate airway remodeling associated with P. aeruginosa.
Recently, epithelial-mesenchymal transition (EMT) has been identified as an important source of fibroblasts that could contribute to the remodeling of the airways. EMT is a reversible process by which epithelial cells change the phenotype and function into that of fibroblast-like, mesenchymal ones, characterized by loss of epithelial markers, acquisition of mesenchymal markers, and matrix metalloproteinase production (Kalluri and Neilson 2003; O’Connor and Gomez 2014). EMT plays an important role in the process of fibrosis (Kim et al. 2006; Flier et al. 2010; Marmai et al. 2011); thus, to reverse EMT process may ameliorate airway fibrotic remodeling. Human bronchial epithelial cells are the first line of defense against invading organisms, which could undergo EMT when exposed to many stimuli (Doerner and Zuraw 2009; Gulino et al. 2016; Polimeni et al. 2016). However, whether EMT in human bronchial epithelial cells contributes to P. aeruginosa–associated airway remodeling is rarely studied.
Transforming growth factor-β1 (TGF-β1) is a pleiotropic growth factor which has been considered a key inducer of fibrosis and has a central role in regulating EMT process by mediating Smad-dependent and Smad-independent signaling pathways (Ask et al. 2008). Previous studies revealed that P. aeruginosa could significantly increase the secretion of TGF-β1 in vivo and in vitro (Yang et al. 2011), which suggested that TGF-β1 may play a critical role in P. aeruginosa–associated airway remodeling. Although the potential therapeutic value of TGF-β1 in fibrotic remodeling, however, blocking of TGF-β1 globally may result in severe side effects as previously reported (Shull et al. 1992; Kulkarni et al. 1993; Dickson et al. 1995). Thus, to regulate TGF-β1 activation selectively by inhibiting activation of TGF-β1, such as epithelium-restricted integrin αvβ6, may represent an ideal strategy.
Integrin αvβ6, an epithelium-restricted transmembrane protein, is expressed at an extremely low level in normal epithelial cells and dramatically increased in response to injury or inflammation stimuli, which could activate endogenous TGF-β1 in a paracrine-like manner. Blocking integrin αvβ6 could inhibit the local activation of TGF-β1 in fibrosis process without systemic side effects (Munger et al. 1999; Horan et al. 2008; Puthawala et al. 2008; Katsumoto et al. 2011). In addition, activation of Smad2/3 signaling pathway is found in fibrogenic process regulated by integrin αvβ6 (Wang et al. 2015). Thus, we hypothesized that EMT may get involved in P. aeruginosa–associated airway fibrotic remodeling, which could be regulated by integrin αvβ6 via activation of TGF-β1-Smad2/3 signaling pathway.
Materials and methods
Cell culture and treatment
BEAS-2B cells derived from the normal human bronchial epithelium were purchased from ATCC and cultured in bronchial epithelial cell growth medium (BEGM, Lonza) at 37 °C in a humidified 5% CO2 atmosphere. Culture flasks should be precoated with a mixture of 0.01 mg/mL fibronectin, 0.03 mg/mL collagen, and 0.001 mg/mL bovine serum albumin dissolved in the medium according to the ATCC recommended protocol. To induce EMT, BEAS-2B cells were seeded at 80% confluence a day before the stimulation of P. aeruginosa’s LPS (Sigma). BEAS-2B cells were pretreated with Integrin αvβ6–blocking antibody (10D5, Abcam) or TGF-β1-Smad2/3 signaling inhibitor, SB431542 (Cell Signaling), for 2 h prior to incubation with 2 μg/mL P. aeruginosa’s LPS (Gong et al. 2014). Morphologic images were captured with an Olympus Inverted Microscope.
Western blotting analyses
Cells were lysed in RIPA buffer (SolarBio, 50 mM Tris/HCl, pH 7.4, 150 mM NaCl, 1% (v/v) NP-40, 0.1% (w/v) SDS) containing 1% (v/v) phenylmethylsulfonyl fluoride (SolarBio), 0.3% (v/v) protease inhibitor (Sigma Aldrich), and 0.1% (v/v) phosphorylated proteinase inhibitor (Sigma) and then clarified by centrifugation. The supernatant was collected and then separated on an SDS-PAGE gel (10% (v/v) polyacrylamide), transferred onto a PVDF membrane. Nonspecific binding was blocked in Tris-buffered saline with Tween 20 (TBS-T) with 8% (w/v) milk for 2 h. After incubation with primary antibodies against β-actin (Abmart), E-cadherin (E-Cad, Abcam), vimentin (Vi, Abcam), α-smooth muscle actin (α-SMA, Sigma), Smad2/3 (Abcam), and p-Smad2/3 (Abcam) for overnight at 4 °C, the membranes were washed with TBS-T for several times and incubated in HRP-linked secondary antibodies (Abmart) for 2 h at room temperature (RT). After repeated washing with TBS-T, the immunoreactive proteins were visualized using enhanced chemiluminescence (Millipore) according to the manufacturer’s instructions and quantified using density analysis, normalized against β-actin and expressed as the fold change compared with the control.
Immunofluorescence
Cells grown on chamber slides were fixed in 4% paraformaldehyde for 30 min at room temperature and permeabilized with 0.1% Triton X-100 at RT for 5 min. The slides were washed with phosphate-buffered saline (PBS) three times and then blocked in 3% bovine serum album (BSA) for 60 min at RT. The cells were incubated with primary antibodies against human E-Cad and Vi (1:100 dilution in PBS with 1% BSA) for 2 h at RT. After several washes with PBS, the slides were incubated with Alexa Fluor 488–conjugated anti-rabbit IgG (Zhongshan Biotechnology, 1:100 dilution in PBS with 1% BSA) for 60 min at RT. After several washes for 15 min with PBS, the slides were incubated with Hoechst 33258 (10 μg/mL) for 10 min at RT. Finally, the slides were washed again, mounting reagent was added, and images were captured using a fluorescence microscope.
ELISA
To determine the secretion of active TGF-β1, metalloproteinase (MMP)-2, and MMP-9, ELISA procedure was performed according to the manufacturer’s instructions (Beijing Rui’erxinde Technology).
Statistical analysis
Data were expressed as mean ± SEM. Significance of the results were analyzed by performing one-way ANOVA, except time-dependent changes of the expression of EMT markers and integrin αvβ6 in BEAS-2B cells which were analyzed with repeated measures ANOVA test. Tukey’s post hoc tests were performed for multiple comparisons. p < 0.05 was considered significant.
Results
P. aeruginosa’s LPS-induced EMT in BEAS-2B cells
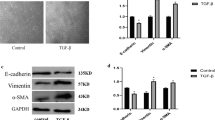
Previous studies demonstrated that BEAS-2B cells could undergo EMT while exposed to many stimuli (Doerner and Zuraw 2009; Gulino et al. 2016; Polimeni et al. 2016); here we showed that P. aeruginosa’s LPS could induce EMT in BEAS-2B cells. After incubation with P. aeruginosa’s LPS (2 μg/mL), the expression of the epithelial marker, E-Cad, and mesenchymal markers, Vi and α-SMA, in BEAS-2B cells were detected using Western blotting at different time points (Fig. 1a). Meanwhile, immunofluorescence was performed to examine the expression of E-Cad and Vi after LPS incubation (Fig. 1b). It was showed that P. aeruginosa’s LPS significantly decreased the expression of the epithelial marker E-Cad and increased the expression of the mesenchymal markers, Vi and α-SMA, in a time-dependent manner by Western blotting. The immunofluorescence assay showed similar changes of E-Cad and Vi expressions in BEAS-2B cells treated with P. aeruginosa’s LPS. Furthermore, we examined morphologic changes in BEAS-2B cells with inverted microscopy (Fig. 1c). The results showed that compared with the control group, a fibroblast-like, spindle-shaped morphology was adopted in BEAS-2B cells incubated with 2 μg/mL P. aeruginosa’s LPS for 72 h. These data indicate that P. aeruginosa’s LPS was sufficient to induce EMT in BEAS-2B cells.
P. aeruginosa’s LPS-induced EMT in BEAS-2B cells. BEAS-2B cells were incubated with 2 μg/mL P. aeruginosa’s LPS for 24, 48, or 72 h. Western blotting revealed that LPS decreased the expression of E-Cad and upregulated the expression of Vi and α-SMA in a time-dependent manner (a). Immunofluorescence showed similar results of the upregulated mesenchymal marker Vi and downregulation of the epithelial marker, E-Cad, in a time-dependent manner (b). Compared with the basal condition, a fibroblast-like, spindle-shaped morphology was induced by P. aeruginosa’s LPS for 72 h in BEAS-2B cells (c). The data represent the mean ± SEM, n = 3. *p < 0.05 versus the control
P. aeruginosa’s LPS increased the expression of integrin αvβ6 in BEAS-2B cells
After incubation with P. aeruginosa’s LPS for 24, 48, 72 h, respectively, integrin β6 expression was examined using Western blotting. The results showed that the expression of β6 integrin was increased in a time-dependent manner (Fig. 2). Because integrin αvβ6 is the only heterodimer which comprise α and β subunits, and the upregulation of β6 is sufficient to increase surface expression of integrin αvβ6 (Niu et al. 2002), it was clear that P. aeruginosa’s LPS could induce an increase of αvβ6 expression in BEAS-2B cells significantly.
The expression of integrin αvβ6 in BEAS-2B cells was significantly upregulated by P. aeruginosa’s LPS in a time-dependent manner. BEAS-2B cells were exposed to 2 μg/mL P. aeruginosa’s LPS for 24, 48, or 72 h. Western blotting revealed that LPS increased the expression of integrin β6 in a time-dependent manner. The data represent the mean ± SEM, n = 3. *p < 0.05 versus the control
Blocking integrin αvβ6 could reverse P. aeruginosa’s LPS-induced EMT in BEAS-2B cells
BEAS-2B cells were pretreated with integrin αvβ6–blocking antibody 10D5 or specific inhibitor of TGF-β1-Smad2/3 signaling, SB431542, at 10 μM for 2 h prior to incubation with LPS for 72 h. The expression of E-Cad, Vi, and α-SMA was detected using Western blotting (Fig. 3a). The expression of E-Cad and Vi was examined by immunofluorescence staining (Fig. 3b). The results showed that the LPS-induced decrease of E-Cad expression was significantly attenuated by integrin αvβ6–blocking antibody 10D5, while the increased expression of Vi and α-SMA was alleviated. These data indicated that blocking of integrin αvβ6 could inhibit the EMT process induced by P. aeruginosa’s LPS in BEAS-2B cells, which provided evidence that integrin αvβ6 plays an important role during P. aeruginosa’s LPS-induced EMT process in human airway epithelial cells. Furthermore, the findings demonstrated that TGF-β1-Smad2/3 signaling pathway may be critical for the pathologic process in BEAS-2B cells which could be inhibited by the specific TGF-β1 inhibitor SB431542.
Blocking integrin αvβ6 could abrogate EMT in BEAS-2B cells induced by P. aeruginosa’s LPS. BEAS-2B cells were incubated with integrin αvβ6–blocking antibody 10D5 (30 μg/mL) or specific inhibitor of TGF-β1-Smad2/3 signaling, SB431542 (10 μM), for 2 h prior to incubation with 2 μg/mL P. aeruginosa’s LPS for 72 h. Western blotting showed that the decrease of the epithelial marker, E-Cad expression, and the increase of mesenchymal markers, Vi and α-SMA, induced by P. aeruginosa’s LPS were reversed by integrin αvβ6–blocking antibody 10D5 as well as SB431542 (a). Immunofluorescence showed similar results of E-Cad and Vi expressions (b). The data represent the mean ± SEM, n = 3. *p < 0.05 versus the control (Con), #p < 0.05 versus LPS
Integrin αvβ6 mediated P. aeruginosa’s LPS-induced EMT in BEAS-2B cells via TGF-β1-Smad2/3 signaling pathway
It has been shown that epithelium-specific αvβ6 could locally regulate the activity of TGF-β1, which mediates EMT and fibrosis via Smad2/3 signaling (Kim et al. 2006; Flier et al. 2010; Marmai et al. 2011). Thus, we examined phosphorylated Smad2/3 using Western blotting and measured the secretion of TGF-β1 using ELISA. P. aeruginosa’s LPS significantly increased the level of phosphorylated Smad2/3. Remarkably, integrin αvβ6–blocking antibody 10D5 could inhibit the increased level of p-Smad2/3 as well as the inhibitor of TGF-β1-Smad2/3 signaling pathway (Fig. 4a). The ELISA showed that increased secretion of active TGF-β1 in P. aeruginosa’s LPS stimulated BEAS-2B cells, which was decreased by αvβ6-blocking antibody 10D5 but not SB431542 (Fig. 4b). These results indicated that P. aeruginosa’s LPS-induced EMT in BEAS-2B cells was mediated by integrin αvβ6 via activation of TGF-β1-Smad2/3 signaling pathway.
Integrin αvβ6 mediated P. aeruginosa’s LPS-induced EMT in BEAS-2B cells via TGF-β1-Smad2/3 signaling pathway. BEAS-2B cells were pretreated with integrin αvβ6–blocking antibody 10D5 (30 μg/mL) or TGF-β1 inhibitor, SB431542 (10 μM), for 2 h prior to incubation with 2 μg/mL P. aeruginosa’s LPS for 72 h. Total-Smad2/3 and p-Smad2/3 were detected using Western blotting (a). The secretion of active TGF-β1 in the culture medium was measured by ELISA (b). The results showed that the phosphorylation of Smad2/3 was increased by P. aeruginosa’s LPS, which were ameliorated by integrin αvβ6–blocking antibody 10D5 as well as SB431542. In addition, elevated secretion of active TGF-β1 was induced by P. aeruginosa’s LPS, which could be inhibited by integrin αvβ6–blocking antibody 10D5. The data represent the mean ± SEM, n = 3. *p < 0.05 versus Con, #p < 0.05 versus LPS
Blocking integrin αvβ6 could inhibit morphologic changes and the increase of MMP-2 and -9 secretion induced by P. aeruginosa’s LPS
Incubated with P. aeruginosa’s LPS at 2 μg/mL for 72 h, BEAS-2B cells adopted a fibroblast-like, spindle-shaped morphology instead of the original cobblestone-shaped appearance. Pretreatment with integrin αvβ6–blocking antibody 10D5 or TGF-β1 inhibitor, SB431542, could prevent the morphologic changes induced by P. aeruginosa’s LPS (Fig. 5a). Increased secretion of active MMP-2 and -9 induced by P. aeruginosa’s LPS treatment was detected using ELISA, which could be attenuated by both 10D5 and SB431542 (Fig. 5b).
Blocking integrin αvβ6 could prevent morphologic changes and the increase of MMP-2 and -9 secretion induced by P. aeruginosa’s LPS. Cell morphologic change was ameliorated by 10D5 and SB431542 (a). ELISA showed that the secretion of active MMP-2 and -9 was significantly increased by P. aeruginosa’s LPS which could be attenuated by integrin αvβ6–blocking antibody 10D5 as well as specific inhibitor of TGF-β1-Smad2/3 signaling, inhibitor SB431542 (b). The data represent the mean ± SEM, n = 3. *p < 0.05 versus Con, #p < 0.05 versus LPS
Discussion
P. aeruginosa is one of the major pathogens in patients with chronic airway diseases, such as cystic fibrosis, chronic obstructive pulmonary disease, bronchiectasis, and bronchiolitis obliterans (Moore and Mastoridis 2017). As a repairing response, airway fibrotic remodeling has been frequently observed in these patients, which could result in irreversible decline of pulmonary function and increased mortality. The mechanism of P. aeruginosa–associated airway remodeling remains unclear. In this study, we demonstrated that EMT could play a crucial role in P. aeruginosa–associated airway remodeling, which is regulated by epithelium-specific integrin αvβ6.
LPS, one of key virulence traits of P. aeruginosa to mediate the interaction between the bacterium and its host (Pier 2007; Raoust et al. 2009), was used to establish an EMT model in BEAS-2B cells. We evaluated EMT in BEAS-2B cells induced by P. aeruginosa’s LPS with multiple approaches to elucidate cell morphologic changes, the expression of epithelial and mesenchymal proteins, and the secretion of MMPs. The results showed that P. aeruginosa’s LPS significantly increased the expression of mesenchymal markers, Vi and α-SMA, accompanied with a decrease of the epithelial marker E-Cad. Meanwhile, BEAS-2B cells stimulated by P. aeruginosa’s LPS changed their cobblestone-like morphology into a fibroblast-like, spindle-shaped morphology. Additionally, increased secretion of MMP-2 and -9 was induced by P. aeruginosa’s LPS in cell medium. Thus, we demonstrated that P. aeruginosa’s LPS is sufficient to induce EMT in human airway epithelial cells. And the findings suggest that EMT is an important source of fibroblasts and plays similar roles during P. aeruginosa–associated airway fibrotic remodeling as it does in pulmonary and renal fibrosis (Hahm et al. 2007).
TGF-β1 has been considered a key inducer of EMT and has a central role in regulating fibrosis process (Kalluri and Neilson 2003; O’Connor and Gomez 2014). Previous studies revealed that P. aeruginosa could significantly increase the secretion of TGF-β1 in vivo (Botha et al. 2008; Vos et al. 2008; Cigana et al. 2016). Our data revealed that P. aeruginosa’s LPS could increase the secretion of TGF-β1 in cell medium of BEAS-2B, which suggested that TGF-β1 may have a critical role in P. aeruginosa–associated airway fibrosis remodeling. Additionally, the level of phosphorylated Smad2/3 was significantly elevated during P. aeruginosa’s LPS-induced EMT in BEAS-2B cells, while SB431542, a selective inhibitor of TGF-β1-Smad2/3 signaling pathway, reversed the changes of EMT markers’ expression and secretion of MMPs as well as morphologic alteration. Previous studies showed that TGF-β1 could induce EMT by mediating Smad-dependent and Smad-independent signaling pathways (Doerner and Zuraw 2009; Gulino et al. 2016; Polimeni et al. 2016); our data indicated that Smad-dependent signaling pathway was the predominant mechanism involved in P. aeruginosa’s LPS-induced EMT in BEAS-2B cells, although Smad-independent signaling pathway may also contribute to the pathologic process.
Even with the critical role of TGF-β1 in the fibrosis process, blocking TGF-β1 globally could increase the risks of severe systemic side effects, such as systemic inflammation, immune disorders, tumors, and even death (Flavell et al. 2010; Seoane and Gomis 2017), which could lead to complicated conditions in infectious patients. It may be an ideal choice to inhibit TGF-β1-Smad2/3 signaling locally for intervention in airway fibrotic remodeling.
Integrin αvβ6 can bind to latency-associated protein of the inactive TGF-β1 complex and provide spatially restricted activation of TGF-β1 (Munger et al. 1999; Horan et al. 2008; Puthawala et al. 2008; Katsumoto et al. 2011). It has been reported that blocking αvβ6 could prevent fibrosis in multiple organs including the lungs without systemic side effects (Wang et al. 2007). Whether integrin αvβ6 is involved in P. aeruginosa–associated airway fibrotic remodeling remains unclear. Our data here showed that P. aeruginosa’s LPS significantly increased integrin αvβ6 expression in BEAS-2B cells, which was consistent with previous finding that the expression of integrin αvβ6 could be dramatically upregulated in response to epithelial cell injury or inflammation (Breuss et al. 1995). Furthermore, we used blocking antibody 10D5 to identify the regulatory effect of integrin αvβ6 on EMT in BEAS-2B cells induced by P. aeruginosa’s LPS. Western blotting revealed that P. aeruginosa’s LPS decreased the expression of the epithelial marker E-Cad and increased the expression of mesenchymal markers, Vi and α-SMA, which could be alleviated by 10D5. The immunofluorescence assay showed similar results of EMT markers’ expression. These data indicated that blocking integrin αvβ6 could attenuate P. aeruginosa’s LPS-induced EMT in BEAS-2B cells, which supports our hypothesis.
Although the studies from Kim and Wang revealed that integrin αvβ6 could regulate EMT in vivo and in vitro via activation of TGF-β1-Smad2/3 signaling pathway (Kim et al. 2006; Flier et al. 2010; Marmai et al. 2011), whether P. aeruginosa–associated airway fibrotic remodeling depends on the interaction between integrin αvβ6 and TGF-β1-Smad2/3 signaling is currently unknown. In this study, BEAS-2B cells were pretreated with 10D5 at 30 μg/mL for 2 h and then incubated with P. aeruginosa’s LPS for 72 h. We detected the secretion of active TGF-β1 using ELISA and measured the phosphorylation level of Smad2/3 using Western blotting, respectively. According to our results, P. aeruginosa’s LPS increased TGF-β1 secretion in cell medium and upregulated the phosphorylated Smad2/3 in BEAS-2B cells, which could be ameliorated by blocking integrin αvβ6. As similar effects of SB431542 were detected on the in vitro model, these results indicated that integrin αvβ6 could regulate P. aeruginosa’s LPS-induced EMT in BEAS-2B cells via activation of TGF-β1-Smad2/3 signaling pathway.
It is noteworthy that toll-like receptor 4 (TLR4), a receptor for LPS which could enhance TGF-β signaling pathway, has been reported to play a key role in the process of organ fibrogenesis and LPS-induced EMT process(Guillot et al. 2004; Seki et al. 2007; He et al. 2016; Tang et al. 2018; Vidya et al. 2018). And interestingly, previous studies implicated that cross talks exist between toll-like receptors and integrin αvβ6(Pittet et al. 2013; Jolly et al. 2014), so integrin αvβ6 might be a therapeutic target for toll-like receptor-mediated fibrogenesis process. Our findings that blocking integrin αvβ6 could ameliorate P. aeruginosa’s LPS-induced EMT in BEAS-2B cells provided another strong evidence for the above hypothesis.
In this study, we also examined whether integrin αvβ6 could regulate morphologic changes and MMP secretion induced by P. aeruginosa’s LPS. Our results showed that P. aeruginosa’s LPS induced BEAS-2B cells to transdifferentiate into a fibroblast-like, spindle-shaped phenotype instead of the original cobblestone-like morphology, which could be alleviated by integrin αvβ6–blocking antibody 10D5. The finding is consistent with a previous study that integrin αvβ6 can regulate morphologic changes during EMT process (Ramos et al. 2009).
MMP-2 and -9 have been demonstrated to be important contributors to fibrosis process by activating TGF-β1 and degrading basement membranes (Corbel et al. 2001; Corbel et al. 2002); here we showed that elevated secretion of MMP-2 and -9 was induced by P. aeruginosa’s LPS, which were significantly attenuated by blocking integrin αvβ6. These data indicated that integrin αvβ6 could regulate MMP-2 and -9 secretion accompanied with EMT in BEAS-2B cells, which was consistent with the studies from Ahmed et al. (2002) and Thomas et al. (2001).
Conclusion
In summary, this study demonstrated that P. aeruginosa’s LPS is sufficient to induce EMT in human bronchial epithelial cells which in turn could contribute to airway fibrotic remodeling. Furthermore, our results obtained here suggest that P. aeruginosa’s LPS-induced EMT in BEAS-2B cells could be regulated by integrin αvβ6–mediated TGF-β1-Smad2/3 signaling activation, and blocking αvβ6 could abrogate EMT changes of BEAS-2B cells, including expression of EMT markers, cell morphology, and secretion of MMP-2 and -9. Thus, integrin αvβ6 might be a safe and effective therapeutic target for P. aeruginosa–associated airway remodeling (Fig. 6).
References
Ahmed N, Pansino F, Clyde R, Murthi P, Quinn MA, Rice GE, Agrez MV, Mok S, Baker MS (2002) Overexpression of alpha(v)beta6 integrin in serous epithelial ovarian cancer regulates extracellular matrix degradation via the plasminogen activation cascade. Carcinogenesis 23:237–244
Almagro P, Salvado M, Garcia-Vidal C, Rodriguez-Carballeira M, Cuchi E, Torres J, Heredia JL (2012) Pseudomonas aeruginosa and mortality after hospital admission for chronic obstructive pulmonary disease. Respiration 84:36–43
Ask K, Bonniaud P, Maass K, Eickelberg O, Margetts PJ, Warburton D, Groffen J, Gauldie J, Kolb M (2008) Progressive pulmonary fibrosis is mediated by TGF-beta isoform 1 but not TGF-beta3. Int J Biochem Cell Biol 40:484–495
Botha P, Archer L, Anderson RL, Lordan J, Dark JH, Corris PA, Gould K, Fisher AJ (2008) Pseudomonas aeruginosa colonization of the allograft after lung transplantation and the risk of bronchiolitis obliterans syndrome. Transplantation 85:771–774
Breuss JM, Gallo J, DeLisser HM, Klimanskaya IV, Folkesson HG, Pittet JF, Nishimura SL, Aldape K, Landers DV, Carpenter W, Et A (1995) Expression of the beta 6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J Cell Sci 108 ( Pt 6:2241–2251
Cigana C, Lore NI, Riva C, De Fino I, Spagnuolo L, Sipione B, Rossi G, Nonis A, Cabrini G, Bragonzi A (2016) Tracking the immunopathological response to Pseudomonas aeruginosa during respiratory infections. Sci Rep 6:21465
Corbel M, Theret N, Caulet-Maugendre S, Germain N, Lagente V, Clement B, Boichot E (2001) Repeated endotoxin exposure induces interstitial fibrosis associated with enhanced gelatinase (MMP-2 and MMP-9) activity. Inflamm Res 50:129–135
Corbel M, Belleguic C, Boichot E, Lagente V (2002) Involvement of gelatinases (MMP-2 and MMP-9) in the development of airway inflammation and pulmonary fibrosis. Cell Biol Toxicol 18:51–61
Dickson MC, Martin JS, Cousins FM, Kulkarni AB, Karlsson S, Akhurst RJ (1995) Defective haematopoiesis and vasculogenesis in transforming growth factor-beta 1 knock out mice. Development 121:1845–1854
Ding C, Yang Z, Wang J, Liu X, Cao Y, Pan Y, Han L, Zhan S (2016) Prevalence of Pseudomonas aeruginosa and antimicrobial-resistant Pseudomonas aeruginosa in patients with pneumonia in mainland China: a systematic review and meta-analysis. Int J Infect Dis 49:119–128
Doerner AM, Zuraw BL (2009) TGF-beta1 induced epithelial to mesenchymal transition (EMT) in human bronchial epithelial cells is enhanced by IL-1beta but not abrogated by corticosteroids. Respir Res 10:100
Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limon P (2010) The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol 10:554–567
Flier SN, Tanjore H, Kokkotou EG, Sugimoto H, Zeisberg M, Kalluri R (2010) Identification of epithelial to mesenchymal transition as a novel source of fibroblasts in intestinal fibrosis. J Biol Chem 285:20202–20212
Gong JH, Cho IH, Shin D, Han SY, Park SH, Kang YH (2014) Inhibition of airway epithelial-to-mesenchymal transition and fibrosis by kaempferol in endotoxin-induced epithelial cells and ovalbumin-sensitized mice. Lab Investig 94:297–308
Guillot L, Medjane S, Le-Barillec K, Balloy V, Danel C, Chignard M, Si-Tahar M (2004) Response of human pulmonary epithelial cells to lipopolysaccharide involves toll-like receptor 4 (TLR4)-dependent signaling pathways: evidence for an intracellular compartmentalization of TLR4. J Biol Chem 279:2712–2718
Gulino GR, Polimeni M, Prato M, Gazzano E, Kopecka J, Colombatto S, Ghigo D, Aldieri E (2016) Effects of chrysotile exposure in human bronchial epithelial cells: insights into the pathogenic mechanisms of asbestos-related diseases. Environ Health Perspect 124:776–784
Hahm K, Lukashev ME, Luo Y, Yang WJ, Dolinski BM, Weinreb PH, Simon KJ, Chun WL, Leone DR, Lobb RR, McCrann DJ, Allaire NE, Horan GS, Fogo A, Kalluri R, Shield CR, Sheppard D, Gardner HA, Violette SM (2007) Alphav beta6 integrin regulates renal fibrosis and inflammation in Alport mouse. Am J Pathol 170:110–125
He Y, Ou Z, Chen X, Zu X, Liu L, Li Y, Cao Z, Chen M, Chen Z, Chen H, Qi L, Wang L (2016) LPS/TLR4 signaling enhances TGF-beta response through downregulating BAMBI during prostatic hyperplasia. Sci Rep 6:27051
Horan GS, Wood S, Ona V, Li DJ, Lukashev ME, Weinreb PH, Simon KJ, Hahm K, Allaire NE, Rinaldi NJ, Goyal J, Feghali-Bostwick CA, Matteson EL, O’Hara C, Lafyatis R, Davis GS, Huang X, Sheppard D, Violette SM (2008) Partial inhibition of integrin alpha(v)beta6 prevents pulmonary fibrosis without exacerbating inflammation. Am J Respir Crit Care Med 177:56–65
Jolly L, Stavrou A, Vanderstoken G, Meliopoulos VA, Habgood A, Tatler AL, Porte J, Knox A, Weinreb P, Violette S, Hussell T, Kolb M, Stampfli MR, Schultz-Cherry S, Jenkins G (2014) Influenza promotes collagen deposition via alphavbeta6 integrin-mediated transforming growth factor beta activation. J Biol Chem 289:35246–35263
Kalluri R, Neilson EG (2003) Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest 112:1776–1784
Katsumoto TR, Violette SM, Sheppard D (2011) Blocking TGFbeta via inhibition of the alphavbeta6 integrin: a possible therapy for systemic sclerosis interstitial lung disease. Int J Rheumatol 2011:208219
Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA (2006) Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci U S A 103:13180–13185
Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S (1993) Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A 90:770–774
Marmai C, Sutherland RE, Kim KK, Dolganov GM, Fang X, Kim SS, Jiang S, Golden JA, Hoopes CW, Matthay MA, Chapman HA, Wolters PJ (2011) Alveolar epithelial cells express mesenchymal proteins in patients with idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 301:L71–L78
Moore JE, Mastoridis P (2017) Clinical implications of Pseudomonas aeruginosa location in the lungs of patients with cystic fibrosis. J Clin Pharm Ther 42:259–267
Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D (1999) The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 96:319–328
Niu J, Dorahy DJ, Gu X, Scott RJ, Draganic B, Ahmed N, Agrez MV (2002) Integrin expression in colon cancer cells is regulated by the cytoplasmic domain of the beta6 integrin subunit. Int J Cancer 99:529–537
O’Connor JW, Gomez EW (2014) Biomechanics of TGFbeta-induced epithelial-mesenchymal transition: implications for fibrosis and cancer. Clin Transl Med 3:23
Pier GB (2007) Pseudomonas aeruginosa lipopolysaccharide: a major virulence factor, initiator of inflammation and target for effective immunity. Int J Med Microbiol 297:277–295
Pittet JF, Koh H, Fang X, Iles K, Christiaans S, Anjun N, Wagener BM, Park DW, Zmijewski JW, Matthay MA, Roux J (2013) HMGB1 accelerates alveolar epithelial repair via an IL-1beta- and alphavbeta6 integrin-dependent activation of TGF-beta1. PLoS One 8:e63907
Polimeni M, Gulino GR, Gazzano E, Kopecka J, Marucco A, Fenoglio I, Cesano F, Campagnolo L, Magrini A, Pietroiusti A, Ghigo D, Aldieri E (2016) Multi-walled carbon nanotubes directly induce epithelial-mesenchymal transition in human bronchial epithelial cells via the TGF-beta-mediated Akt/GSK-3beta/SNAIL-1 signalling pathway. Part Fibre Toxicol 13:27
Puthawala K, Hadjiangelis N, Jacoby SC, Bayongan E, Zhao Z, Yang Z, Devitt ML, Horan GS, Weinreb PH, Lukashev ME, Violette SM, Grant KS, Colarossi C, Formenti SC, Munger JS (2008) Inhibition of integrin alpha(v)beta6, an activator of latent transforming growth factor-beta, prevents radiation-induced lung fibrosis. Am J Respir Crit Care Med 177:82–90
Ramos DM, Dang D, Sadler S (2009) The role of the integrin alpha v beta6 in regulating the epithelial to mesenchymal transition in oral cancer. Anticancer Res 29:125–130
Raoust E, Balloy V, Garcia-Verdugo I, Touqui L, Ramphal R, Chignard M (2009) Pseudomonas aeruginosa LPS or flagellin are sufficient to activate TLR-dependent signaling in murine alveolar macrophages and airway epithelial cells. PLoS One 4:e7259
Sanchez-Munoz G, Lopez DAA, Jimenez-Garcia R, Carrasco-Garrido P, Hernandez-Barrera V, Pedraza-Serrano F, Puente-Maestu L, de Miguel-Diez J (2016) Time trends in hospital admissions for bronchiectasis: analysis of the Spanish national hospital discharge data (2004 to 2013). PLoS One 11:e162282
Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF (2007) TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med 13:1324–1332
Seoane, J. and R. R. Gomis (2017). "TGF-beta Family Signaling in Tumor Suppression and Cancer Progression." Cold Spring Harb Perspect Biol9(12).
Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, Et A (1992) Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature 359:693–699
Tang S, Jiang X, Wu L, Chen S, Chen L, Jiang J, Yan P, Wang F, Tu K, Wang D, Gu J, Zhao L (2018) Toll-like receptor 4 shRNA attenuates lipopolysaccharide-induced epithelial-mesenchymal transition of intrahepatic biliary epithelial cells in rats. Biomed Pharmacother 107:1210–1217
Thomas GJ, Poomsawat S, Lewis MP, Hart IR, Speight PM, Marshall JF (2001) Alpha v beta 6 integrin upregulates matrix metalloproteinase 9 and promotes migration of normal oral keratinocytes. J Invest Dermatol 116:898–904
Vidya MK, Kumar VG, Sejian V, Bagath M, Krishnan G, Bhatta R (2018) Toll-like receptors: significance, ligands, signaling pathways, and functions in mammals. Int Rev Immunol 37:20–36
Vos R, Vanaudenaerde BM, Geudens N, Dupont LJ, Van Raemdonck DE, Verleden GM (2008) Pseudomonal airway colonisation: risk factor for bronchiolitis obliterans syndrome after lung transplantation? Eur Respir J 31:1037–1045
Wang B, Dolinski BM, Kikuchi N, Leone DR, Peters MG, Weinreb PH, Violette SM, Bissell DM (2007) Role of alphavbeta6 integrin in acute biliary fibrosis. Hepatology 46:1404–1412
Wang J, Bao L, Yu B, Liu Z, Han W, Deng C, Guo C (2015) Interleukin-1beta promotes epithelial-derived alveolar elastogenesis via alphavbeta6 integrin-dependent TGF-beta activation. Cell Physiol Biochem 36:2198–2216
Yang JJ, Wang DD, Sun TY (2011) Flagellin of Pseudomonas aeruginosa induces transforming growth factor beta 1 expression in normal bronchial epithelial cells through mitogen activated protein kinase cascades. Chin Med J 124:599–605
Zhuo H, Yang K, Lynch SV, Dotson RH, Glidden DV, Singh G, Webb WR, Elicker BM, Garcia O, Brown R, Sawa Y, Misset B, Wiener-Kronish JP (2008) Increased mortality of ventilated patients with endotracheal Pseudomonas aeruginosa without clinical signs of infection. Crit Care Med 36:2495–2503
Acknowledgments
We would like to thank the Key Laboratory of Geriatrics of Beijing Institute of Geriatrics, Beijing Hospital Ministry of Health, for providing excellent facilities to conduct our experimental studies.
Funding
The work was supported by grants from projects supported by National Natural Science Foundation of China (81370101).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Liu, W., Sun, T. & Wang, Y. Integrin αvβ6 mediates epithelial-mesenchymal transition in human bronchial epithelial cells induced by lipopolysaccharides of Pseudomonas aeruginosa via TGF-β1-Smad2/3 signaling pathway. Folia Microbiol 65, 329–338 (2020). https://doi.org/10.1007/s12223-019-00728-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-019-00728-w