Abstract
Chilling (Ch) and salinity (S) are challenging stresses affecting plant physiology, growth, and productivity. The current study investigated the effects of these two stresses, singly and in combination, on photosynthetic performance and ultrastructure of chloroplast of faba beans (Vicia faba L. Cv. Aspani). Plants were exposed to 3 °C and 120 mM NaCl for 16 h in an optimized soil mixture (sand:clay 2:1) under optimized conditions. Results showed that both Ch and S significantly reduced photosynthetic rates, Fv/Fm, chlorophyll content, stomatal index, and stomatal conductance. Chilling caused changes in chloroplast ultrastructure (swelling, ruptured envelopes, and shrunk lamellae), while salinity caused more deformation of the thylakoid membrane and disorganization of the grana structure. However, there was an antagonistic effect between Ch x S. The tolerance of plant to 120 mM NaCl, in the present study, was improved by exposure to Ch which rather allowed the maintenance of chloroplast ultrastructure and morphology of stomata. Moreover, using SEM and TEM gave an effective insight of the ultrastructural damage in plant cells under stress and helps to consider the underlying mechanisms of stress effects. Our results suggest that Ch mitigates the noxious effect of S on the photosynthetic performance of Vicia faba plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Plants are continually exposed to a variety of environmental stresses (Hassan et al. 2021a; Nasser et al. 2022). Plants develop a wide range of biochemical and molecular mechanisms to combat such stresses Hassan et al. 2021b). An intriguing field of study is the molecular and genetic underpinnings of stress response signaling in plants (Hassan et al. 2017; El Maghraby and Hassan 2021).
Salinity and chilling (0–5 °C) are the two main abiotic stresses for bean seed production (Srivastava et al. 2015; Liu et al. 2018; Torche et al. 2018). The rising salinization of the soil and the excessive use of low-quality water for irrigation are both pressures that limit the growth and productivity of many plants worldwide (Al Maleebi et al. 2014; Srivastava et al. 2015).
The response to cold stress is a very complicated process that involves both physiological and biochemical changes (such as altered protein patterns and enzyme activity, altered photosynthetic patterns, and changes in the ultrastructure of cellular organelles) (Younis et al., 2011). In addition, one of the main problems restricting plant growth and yield, and modifying metabolic and physiological processes, particularly in arid and semi-arid climates, is salt stress (Abdel Mageed et al. 2016).
Recently, Hammed et al. (2021) stated that salinity is a growing problem worldwide, disrupting metabolic processes, and reducing the development, growth, and yield of crops. Salinity can alter the chloroplast size, number, and lamellar organization. However, normal chloroplast physiology should be maintained for the survival of the entire plant (Oi et al. 2020).
Water deficiency and osmotic stress induction are two effects of salinity stress. However, low temperatures may also lead to water deficiency at the cellular level because of a lack of root water conductance and restricted root activity (Bazrafshan et al. 2020). Furthermore, the alteration of metabolic pathways brought on by cold stress, salt, and drought results in the generation of reactive oxygen species (ROS), which cause oxidative damage to plants (Srivastava et al. 2015).
Chilling stress causes a restriction in CO2 fixation, generating ROS, which causes the oxidation of cellular constituents and compromises the integrity of the chloroplasts (Fuji et al., 2017). ROS’s effects under salinity stress included a reduction in stomatal opening, disorder of the chloroplast’s thylakoid ultrastructure, and a reduction in photosynthetic rates (Rahman et al. 2016; Jalil et al. 2020).
Under drought stress, the effects included a reduction in chlorophyll content and CO2 assimilation (Ansari and Lin 2010). Therefore, changes in the lamellar organization might cause chloroplast shrinkage and an unrecognizably irregular grana structure as a result of environmental variables altering the chloroplast structure.
The physiological, metabolic, and cellular alteration symptoms of chilling injury and salinity are controlled by the duration of exposure, the species, the cultivar, and the developmental stage (Younis 2021). It is a worth to mention that bean grows throughout the winter (November to January), so it may suffer from chilling injury during cold seasons.
Faba beans are one of the most widely consumed traditional foods in many Mediterranean countries, and Egyptians heavily rely on them in their diet, due to its great nutritional content (Zeidan 2002). In addition, legumes are crucial to sustainable agriculture (DeCillis et al. 2019).
Although the interaction effects of salinity with many environmental stresses have been extensively studied, its interaction with chilling is extremely lacking (Al-Shoaibi et al. 2021).
The present study aimed to elucidate and evaluate the impact of chilling and salinity, both separately and together, on the photosynthetic performance and chloroplast ultrastructure of Vicia faba L. Cv. Aspani. This study, to the best of our knowledge, is the first of its kind to investigate the response of beans to these stresses.
2 Materials and methods
Seeds of the faba bean (V. faba L. Cv. Aspani) were purchased from a commercial source in Egypt. They were soaked in distilled water for 24 h to break dormancy, and then they were treated with a very diluted chlorine solution for 5 min for sterilization and to remove pesticides. Finally, then they were washed with distilled water three times (Hassan et al. 2018; Baqasi et al. 2018).
The experiment was carried out under optimized conditions, which included a Photon flux density (PFD) of 375 µmol m−2 s−1, a 10/14 h light/dark cycle, a temperature of 20 ± 2°C, and a relative humidity level of 85%. The field capacity of the culture pots was estimated at 200 mL of demineralized water.
Five faba seeds, that had been pre-soaked, were placed 1 cm beneath the soil’s surface in plastic pots (15 cm diameter and 15 cm height), and were left to germinate for 10 days at room temperature. Pots were filled with a well-homogenous soil mixture (clay: sand 1:2). Seeds were watered twice a week with distilled water at field capacity till the first trifoliate leaf appeared (10 days after sowing “DAS”). Seedlings were watered with a Hoagland solution (0.25 full-strength) 10 DAS (Hassan et al. 2021a).
Pots were divided into two groups,14 DAS: one group received distilled water while the other group received 120 mM NaCl for 48 h; both groups were exposed to room temperature (22 °C), in closed growth chambers. There were 40 pots (10 pots/treatment), and they were distributed among treatments in a randomized block design (RBD). The pots were divided into four groups,16 DAS: one was incubated at 3 °C and irrigated with distilled water (Ch); the second one was incubated at room temperature and irrigated with 120 mM NaCl (S); the third group was incubated at 3 °C and irrigated with 120 mM NaCl (Ch x S); the fourth group was left as a control to grow at room temperature and irrigated with distilled water (C).
2.1 Non-destructive harvests
Net photosynthetic rates and stomatal conductance were assessed 4 and 16 h following exposure to both stressors. However, chlorophyll fluorescence was measured, only once, after 16 h of exposure.
2.2 Measurements of gas exchange parameters
Total CO2 stomatal conductance (gs) and net photosynthetic rate (A) of the second true trifoliate leaf of each plant/treatment were measured with an open infrared gas exchange system under a PDF of 375 µmol m−2 s−1 and a CO2 concentration of 405 ppm (LI- 6400, LICOR, Lincoln, NE, USA (Ismail et al. 2014; Basahi et al. 2016; Hassan et al. 2018). Measurements were carried out at 4 and 16 h following exposure to both stressors.
2.3 Determination of chlorophyll fluorescence
Chlorophyll fluorescence measurements were carried out with a portable photosynthetic efficiency analyzer (PEA, Hansatech Instrumental, Hardwick, Norfolk, UK) on the same leaves used for gas exchange at the end of the experiment (16 h following exposure to both stressors). Leaves were dark-adapted for 30 min using a dark leaf clip. The minimal (F0), maximum (Fm), and variable (Fv) chlorophyll fluorescence were recorded to calculate the optimal quantum efficiency of PSII photochemistry [Fv/Fm = (Fm–F0)/Fm] (Ismail et al. 2014; Basahi et al. 2016).
The light and dark fluorescence parameters were used for calculations of the photochemical (qP) and non-photochemical (NPQ) quenching coefficients (Hassan et al. 2021b).
2.4 Destructive harvests
Plants were destructively harvested at the end of exposure to both stresses (16 h) to measure chlorophyll content. In addition, leaves from the last treatment were prepared for scanning electron microscopy (SEM) and transmission electron microscope (TEM) in order to examine change in the chloroplast ultrastructure.
2.5 Determination of chlorophyll content
Chlorophyll was extracted from three leaves on the main stems of plants per treatment. The chlorophyll concentration was determined (Ismail et al. 2014).
At harvest, fresh leaves were collected to measure chlorophyll content. Chlorophyll was extracted from fresh leaves (5 plants/treatments) using 80% 200 (v/ v) cold acetone from 0.1 g of chopped fresh leaves under low light or dark conditions. Absorbance readings were taken with an ultraviolet spectrophotometer (UV-2450, SHIMADZU, Japan) at 645 and 663 nm, and a commercial chemical detection kit (Suzhou Gris Biotechnology Co., LTD). The formulas provided by Suzhou, China was used calculate the contents of total chlorophyll (Tot Chl), chlorophyll a (Chl a) and chlorophyll b (Chl b) (El Dakak and Hassan 2020).
2.6 Measurements of stomatal attributes
Patches of 1 cm2 in size around the midpoint at the lower surface of the leaf blade were excised from the second leaflet to determine the stomatal density. It was calculated from ten fully expanded leaves per treatment at × 400 magnification using a “RITZ Table Top Light Microscope”.
Stomatal index (%) was calculated according to the following equation:
where SI% is the stomatal index percentage, S is the number of stomata cells per mm2, and E is the number of epidermal cells per mm2 (Roy 2010; Hameed et al. 2021).
2.7 Scanning electron microscopy (SEM)
Tissue samples were taken from different treatments and prepared using the glutaraldehyde/osmium/critical point drying technique and examined using SEM (Philips Model 500). Stomata were counted on the abaxial surface of each leaf/treatment (ten fields/leaf) and were classified as either opened or closed. The length and width of stomata were measured in (µm) (Hassan et al. 1994, 2017; Taia et al. 2013).
2.8 Transmission electron microscopy (TEM)
Small (1 mm2) segments of the center of the fully expanded uppermost leaflet were cut off and fixed for 3 h at room temperature using 3% glutaraldehyde in a solution containing 100 mM Sodium Phosphate, pH 7 at 5 °C (Li et al. 2013). After the samples had been post-treated in 2% OsO, they were washed once more with buffer for 5 h at room temperature, and then they were dried in acetone and embedded in Epon resin. The samples were cut into ultra-sections of approximately 50–70 µm with a LKB-V ultramicrotome and stained with uranyl acetate and lead phosphate, and then they were examined by a transmission electron microscope (JEM-1230 JEOL, Japan) (Gao et al. 2015).
2.9 Statistical analysis
A three-way analysis of variance (ANOVA), using salinity, low temperature and time as factors, using the SAS Statistical Package (SAS software, Version 9.1, SAS Institute, Cary, North Carolina, USA). Data were log-transformed prior to analysis to ensure that they were normally distributed. PPFD was used as a covariate in the ANOVA of gas exchange measurements. The least significant differences (LSDs) were used to identify the significant differences between treatments at P ˂ 0.05. Data were presented as means ± standard deviation (n = 3).
3 Results
3.1 Effect of chilling and salinity stressors on g s and A
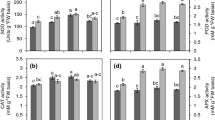
Ch or S reduced gs by 13.6 and 50%, respectively, after 4 h of exposure, and by 25.8 and 48.1% after 16 h of exposure, respectively (Fig. 1A). Similarly, A was decreased by 16.9 and 41.4% after 4 h, and by 20.4 and 43% after 16 h of exposure, respectively (Fig. 1B).
Interestingly, both gs and A of were improved after 4 h exposure to Ch x S by 31.8 and 25%, and after 16 h of exposure by 35.2 and 28.8%, respectively.
3.2 Effect of chilling and salinity on chlorophyll fluorescence
Chlorophyll fluorescence of dark-adapted broad bean leaves is presented in Table 1. Variable to maximal fluorescence ratio (Fv/Fm), photochemical quenching coefficient (qP), and non-photochemical quenching (NPQ) were significantly altered due to exposure to chilling and/or salinity stresses. Fv/Fm and qP were decreased significantly by 18.3% and by 23.7% due to exposure to Ch and S, respectively. Interactive effects of both stresses was less than additive, and it caused a reduction in theses parameters by 19.4% each (Table 1). Non-photochemical quenching (NPQ) was increased significantly by 90%, onefold, and 94% in leaves exposed to Ch, S, and Ch x S, respectively.
3.3 Effect of chilling and salinity on chlorophyll
The influence of chilling and salinity singly and in combination after 16 h exposure on chlorophyll pigments content is presented in Table 2. There was a significant reduction in chl a, Chl b, and chl (a + b) content in the chilled plants by 19.3, 27.7, sand 21%, respectively. Similarly, salinity reduced these parameters by 35.5, 27.7, and 38%, respectively. Interactive effects of Ch and S caused reductions in these parameters by 23.9, 36.1, and 28.6%, respectively. However, chlorophyll a/b increased by 5.7, 20.8, and 18.9% due to Ch, S, and Ch x S, respectively.
3.4 Effect of chilling and salinity on stomatal parameters
It was found that SI% was decreased significantly by 15% and 67% due to exposure to chilling and salinity, respectively (Table 3). Interactive effects of chilling and salinity was less than salinity additive; it reduced SI% by 33%.
Chilling reduced percentage of opened stomata by 35% and stomatal width by 45% (Table 3), while salt stress caused reductions in these parameters by 78 and 72%, respectively. Ch x S resulted in 59 and 51% reductions in both parametres, respectively.
Scanning electron micrographs of bean leaves exposed to Ch and/or S are illustrated in Fig. 2. Epidermal cells of plants exposed to chilling showed collapsed and shrunk cell walls (Fig. 2B), while those exposed to salinity showed more collapsed and shrunk walls (Fig. 2C).
Figure 3 shows a magnified scanning micrographs. Chilling or salinity stress caused wrinkling and shrinkage of epidermal cells which led to stomatal closure (Fig. 3B, 3 C). Ch x S caused wrinkling of epidermal cells but at a lower extent accompanied by partially opened stoma (Fig. 3D).
3.5 Effect of chilling and salinity on ultrastructure
TEM revealed that chilling caused swelling of chloroplast, rupture of chloroplast envelope, and shrinkage of lamellae (Fig. 4B). Salinity caused change in the chloroplast size, chloroplast bursting and distortion, and disappearance of starch granules (Fig. 4C). Exposure to both stresses was antagonistic as chloroplast maintained their shapes and integrity in a way like the control plants (Fig. 4D).
4 Discussion
Photosynthetic activities are good indicators of environmental stresses (Kalisz et al. 2016). Exposure to chilling and salinity (short- or long-term) imposes different impact on crops (Ljubej et al. 2021; Liu and Zhang 2020).
Salinity level determines the suitability of low-quality water irrigation for crops, and it also represents one of the major stressors to the growth and productivity of the highly important economic crops (Bimurzayev et al. 2021). Water deficit and osmotic stress at the cellular level is a common consequence of salinity as well as chilling stress when they are solely existed (Bazrafshan et al. 2020). Thus, it was postulated that drought stress would exacerbates the problem when both stresses combined under the prevailing conditions (Bazrafshan et al. 2020).
Enhancement of the CO2 influx to leaves through motivating stomatal conductance could improve photosynthetic rate and metabolites accumulation (Li et al., 2014b). Therefore, the reduction in photosynthetic rate is mostly attributed to the loss in stomatal conductance (Lawlor and Cornic 2002).
In the present study, exposure to Ch or S for 4 and 16 h, adversely affect the physiology of bean plants, where salinity showed more noxious effect on net photosynthetic rate and stomatal conductance than chilling response. Stomatal closure as a first response to salinity leads to lessen of stomatal conductance, transpiration rate and photosynthetic rate. Moreover, under salt stress, significant decreases in attributes of photosynthetic rate, chlorophyll fluorescence, stomatal conductance and rate of electron transport were recorded for the wild-type of V. faba L. compared to the normal growth conditions (Desouky et al. 2021).
Interactive Ch x S showed a better effect than Ch or S alone. Moreover, Ch causes lower negative effects than S or Ch x S. Therefore, we could argue that Ch could mitigate the deleterious effect of S when both stresses occur simultaneously.
Chlorophyll fluorescence (Fv/Fm) is the most powerful and widely used techniques to study the effect of stresses on the photosynthetic performance, expressing the efficiency of PSII (Hosseinzadeh et al. 2016).
Lower values of Fv/Fm in plants exposed to Ch and/or S suggests that a proportion of the photosystem II reaction centers are inactivated or damaged (Zhou et al. 2018; Badr 2020). Inhibition of Fv/Fm was associated with PSII photodamage, in olives, under saline conditions (Loreto et al. 2013). Similarly, increases in salinity levels led to parallel reductions in PSI and PSII activities in four rice cultivars (Tiwari et al. 2008).
Reduction in chlorophyll content is another consequence of cold stress, leading to photosynthetic disruption. Chilling exerts a significant negative impact on chlorophyll pigments content and their ratios in the present studied V. faba. Cholakova and Vassilev, (2017) showed that chlorophyll content of Zea mays showed a significant reduction under chilling stress, and they attributed these effects to chlorophyll photooxidative damage. The inhibition in chlorophyll content due to exposure to 120 mM NaCl in the present study is in agreement with the results of Dawood and El-Awadi, (2015) on beans and Taffouo et al. (2010) on Vigna subterranean. Reduction of chlorophyll content (either by fast degradation or lessen biosynthesis) may be used as a tool by plants to increase the capacity for photoprotection mechanism to limit light absorbance under stress conditions (ElSheery and Cao 2008). Moreover, the reduction in chlorophyll content was attributed to the role of chlorophyllase enzyme that impairs chlorophyll biosynthesis and accelerates its degradation (Santos 2004). However, during degradation of chlorophyll, Chl b may be converted into Chl a resulting in the increased content of Chl a, thus higher ratio of Chl a/b (Eckardt 2009).
Stomata are the port for gas exchange control; thus, for optimizing water use efficiency and minimize transpiration rate, it is pertinent to allow changes in stomatal size and density. Stomatal closure is known to be induced by cold temperature, drought stress, darkness, high CO2, and plant hormones/elicitors (Kim et al. 2010). Chilling caused significant reduction in stomatal index%, stomatal width, percentage of opened stomata, accompanied by wrinkling and shrinkage epidermal walls; this is in agreement with the results of Wilkinson et al. (2010), who reported that exposure to low temperature enhanced stomatal closure in Commelina communis.
Plants restrict stomatal opening as a short-term response to control their transpiration rate in order to alleviate effects of salt stress. This is in agreement with our results as stomatal index% and stomatal width were significantly reduced under salinity stress.
The differential stomatal response reveals that there might be an inherent collaboration of the physiological processes (e.g., gas exchange) and morphological traits (e.g., stomatal width and % of opened stomata) to regulate the gas exchange and transpiration rate when bean plants exposed to unfavorable conditions. Gago et al (2016) reported that the balance in the level of sugars and organic acids may regulate stomatal movements through a complex metabolic network and, consequently, photosynthesis. However, the identification of transporters involved in importing metabolites to guard cell was beyond the scope of the present work, and this warrants a further exploration. Moreover, we have to study when (in which period of the day, which period of stress, and in response to which stimulus) and where (apoplast, symplast, vacuole, chloroplast, and mitochondrion) the metabolites would be accumulated to regulate stomatal movements.
Though environmental stress may have direct effects on stomatal characteristics that cause a decrease in photosynthetic performance, non-stomatal factors could also have pertinent roles in restricting photosynthesis (El-Dakak and Hassan 2020). Therefore, reduction in photosynthetic rates could occur due to stomatal and/or non-stomatal factors (Hassan et al. 2021a, b). The closure of stomata results in a shortage of CO2. Non-stomatal factors could include an increase in diffusive resistance to CO2 in the mesophyll; a reduction in PSII; and a decrease in chlorophyll content and inhibition of electron transport (Goussi et al. 2018).
Chloroplast is the house of most physiological processes relevant to photosynthesis. Research has shown that keeping the physiology of the normal chloroplast away from stress is a need for the persistence of the entire plant. Ultrastructure of chloroplast is the first harshly affected in crop plants subjected to chilling injury (Kratch and Wise 2000). Salinity leads to structural and ultrastructural effects, particularly in salt-sensitive species. Some of them are considered injury onset marker, such as: chloroplasts aggregation coupled with granal swelling and fret compartments or the complete deformation of chloroplastic grana and thylakoid structures (Goussi et al. 2018). This effects could to be attributed to photooxidative damage generated during chilling (Goussi et al. 2018).
Ultrastructure chloroplasts in the current study revealed that exposure to chilling led to swelling of chloroplast, shrinkage of lamellae and rupture of chloroplast's envelope. Salinity also induced ruptured envelope, more deformation of thylakoid membrane and distortion of granal organization.
The inhibition in PSII (Fv/Fm) is a good indicator of the photodamage in chloroplasts, especially in thylakoid membranes, under salinity or chilling stress (Yamane et al. 2008).
The photodamage and partial inactivation of PSII and PSI reaction centers in thylakoids cause loss in chlorophyll content; this is due to the photoinhibition and their subsequent ROS production under the prevailed stressful conditions. The significant inhibition in net photosynthetic rate is influenced by reduction in stomatal conductance and destruction of stomatal morphology (wrinkling and shrinkage of epidermal cells that adjacent to stomata). It appears that both SEM and TEM helped to unveil how plants responded to Ch and/or S.
5 Conclusions
An interesting area of research is to, constantly, monitor plants survival when they encounter a complex range of abiotic stresses.
Either chilling or salinity stress promoted limitations in several physiological attributes. Nevertheless, V. faba L. Cv. Aspani has triggered some mechanisms to mitigate the toxic effect of salinity by chilling. These mechanisms related to stomatal and/or non-stomatal factors which deal with this constraint throughout the maintenance of stomatal parameters, stomatal conductance, gas exchange, chlorophyll content, chlorophyll fluorescence, PSII efficiency, and net photosynthetic rate. The tolerance of plant to 120 mM NaCl, in the present study, was improved by exposure to Ch which rather allowed the maintenance of chloroplast ultrastructure and morphology of stomata. Moreover, using SEM and TEM gave an effective insight of the ultrastructural damage in plant cells under stress and helps to consider the underlying mechanisms of stress effects.
Data availability
All data generated or analyzed during this study are included in this manuscript.
References
Abd El-Mageed TA, Semida WM, Howladar SM, Zaki SS, Rady M (2016) Hydrogen peroxide improves the antioxidant defence system in salt stressed. Allium Cepa Plants. 4:91–100
Al Meelebi T, Ismail I, Basahi J, Qari HA, Hassan IA (2014) Hazardous of wastewater irrigation on quality attributes and contamination of citrus fruits. Biosci Biotech Res 11:89–97
AL-Shoaibi, A. A. (2021) Interactive effects of salinity and chilling stress on the growth of the two forage species elephant grass and maize. Egypt J Bot 61:579–590
Ansari MI, Lin TP (2010) Molecular analysis of dehydration in plants. Int Res J Plant Sci. 1:21–25
Badr G (2020) Combined effects of vermicompost and salinity on growth, biochemical and molecular traits of Vicia Faba. Thesis, Alexandria University, Alexandria, Egypt, M.Sc
Baqasi LA, Qari HA, Nahhas NA, Badr RH, Taia WK, El Dakak R, Hassan IA (2018) Effects of low concentrations of O3 on metabolic and physiological attributes in wheat (Triticum aestivum L.) plants. Biomed Pharmacol 11:929–934
Basahi JM, Ismail I, Haiba NS, Hassan IA, Lorenzini G (2016) Assessing ambient ozone injury in olive (Olea europaea L.) plants by using the antioxidant ethylene diurea (EDU) in Saudi Arabia. Enviro Monit Assess 188:1–11
Bazrafshan A, Shorafa M, Mohammadi MH, Zolfaghari AA, van de Craats D, van der Zee SE (2020) Comparison of the individual salinity and water deficit stress using water use, yield, and plant parameters in maize. Environ Monit Assess 192:448
Bimurzayev N, Sari H, Kurunc A, Doganay KH, Asmamaw M (2021) Effects of different salt sources and salinity levels on emergence and seedling growth of faba bean genotypes. Sci Rep 11:18198
Cholakova-Bimbalova R, Vassilev A (2017) Effect of chilling stress on the photosynthetic performance of young plants from two maize (Zea mays) hybrids. CBU Int Conf Proc 5:1118–1123
Dawood MG, El-Awadi ME (2015) Alleviation of salinity stress on Vicia faba L. plants via seed priming with melatonin. Acta Biol Colomb 20:223–235
De Cillis F, Leoni B, Massaro M, Renna M, Santamaria P (2019) Yield and Quality of Faba Bean (Vicia faba L var major) genotypes as a vegetable for fresh consumption: a comparison between Italian landraces and commercial varieties. Agriculture 9:253
Desouky AF, Ahmed AHH, Stützel H, Jacobsen H-J, Pao Y-C, Hanafy MS (2021) Enhanced abiotic stress tolerance of Vicia faba L. plants heterologously expressing the PR10a Gene from potato. Plants 10:173–179
Eckardt NA (2009) A new chlorophyll degradation pathway. Plant Cell 21:700–711
El Maghraby D, Hassan IA (2021) Photosynthetic and biochemical response of Ulva lactuca to marine pollution by polyaromatic hydrocarbons (PAHs). Egypt J Bot 61:467–478
El-Dakak R, Hassan IA (2020) The Alleviative effects of salicylic acid on physiological indices and defense mechanisms of maize (Zea Mays L. Giza 2) stressed with cadmium. Environ Processes 7:873–884
Elsheery NI, Cao KF (2008) Gas exchange, chlorophyll fluorescence, and osmotic adjustment in two mango cultivars under drought stress. Acta Physiol Plant 30:769–777
Gago J, Daloso D, Carlos Figueroa M, Flexas J, Fernie AR, Nikoloski Z (2016) Relationships of Leaf Net Photosynthesis, Stomatal Conductance, and Mesophyll Conductance to Primary Metabolism: A Multispecies Meta-Analysis Approach. Plant Physiol 171:265–279. https://doi.org/10.1104/pp.15.01660
Gao H-J, Yang H-Y, Bai J-P, Liang X-Y, Lou Y, Zhang J-L, Wang D, Zhang J-L, Niu S-Q, Chen Y (2015) Ultrastructural and physiological responses of potato (Solanum tuberosum L.) plantlets to gradient saline stress. Front Plant Sci 5:787–794
Goussi R, Manaa A, Derbali W, Cantamessa S, Abdelly C, Barbato R (2018) Comparative analysis of salt stress, duration and intensity, on the chloroplast ultrastructure and photosynthetic apparatus in Thellungiella salsuginea. J Photochem Photobiol B 183:275–287
Hameed A, Ahmed MZ, Hussain T, Aziz I, Ahmad N, Gul B, Nielsen BL (2021) Effects of salinity stress on chloroplast structure and function. Cells 10:2023
Hassan IA, Ashmore MR, Bell JNB (1994) Effects of O3 on the stomatal behavior of Egyptian varieties of radish (Raphanus sativus L cv Baladey) and turnip (Brassica rapa L cv Sultani). New Phytol 128:243–249
Hassan IA, Haiba N, Badr R, Basahi JM, Almeelbi T, Ismail IM, Taia WK (2017) Effects of ambient ozone on reactive oxygen species and antioxidant metabolites in leaves of pea (Pisum sativum l.) Plants. Pak J Bot 49:47–55
Hassan IA, Bell JNB, Ashmore MR, Ismail IM, Haiba NS, Almeelbi T, Basahi JM (2018) Radish (Raphanus sativus L.) cultivar-specific response to O3: the patterns of biochemical and plant growth characteristics. Clean-Soil Air Water 46:1–9
Hassan IA, El Dakak R, Haiba NS, Abd Elmegeid W, El-Sheekh M, Abdul Rahman S, Basahi J, Summan A, Ismail I (2021a) Biochemical and physiological response of rice (Oryza sativa L.) plants to copper oxide nanoparticle stress. Agrochimica 66:54–67
Hassan IA, Sayegh FS, El-Sheekh MM, Walter JW, El Maghraby DM (2021b) Interactive effects of salinity and copper toxicity on the growth and photosynthetic efficiency of germlings and adult brown alga Fucus ceranoides Fucales. Rend Fis Acc Lincei 32, 737–745
Hosseinzadeh SR, Amiri H, Ismaili A (2016) Effect of vermicompost fertilizer on photosynthetic characteristics of chickpea (Cicer arietinum L.) under drought stress. Photosynthetica 54:87–92
Ismail IM, Basahi JM, Hassan IA (2014) Gas exchange and chlorophyll fluorescence of pea (Pisum sativum L.) plants in response to ambient O3 at a rural site in Egypt. Sci Total Environ 497–498:585–593
Jalil SU, Ansari MI (2020) Physiological role of Gamma-aminobutyric acid in salt stress tolerance. In: Hasanuzzaman M (ed) Salt and Drought Stress Tolerance in Plants. Cham, Switzerland, Springer Nature
Kalisz A, Jezdinský A, Pokluda R, Sękara A, Grabowska A, Gil J (2016) Impacts of chilling on photosynthesis and chlorophyll pigment content in juvenile basil cultivars. Hortic Environ Biotechnol 57:330–339
Kim TH, Bohmer M, Hu HH, Nishimura N, Schroeder JI (2010) Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Ann Rev Plant Biol 61:561–591. https://doi.org/10.1146/annurev-arplant-042809-112226
Lawlor D, Cornic G (2002) Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ 25:275–294
Liu L, Zhang X (2020) Effects of temperature variability and extremes on spring phenologyacross the contiguous United States from 1982 to 2016. Sci Rep 10:17952
Liu X, Zhou Y, Xiao J, Bao F (2018) Effects of chilling on the structure, function and development of chloroplasts. Front Plant Sci 9:1715
Ljubej V, Karalija E, Salopek-Sondi B, Šamec D (2021) Effects of short-term exposure to low temperatures on proline, pigments, and phytochemicals level in kale (Brassica oleracea var. acephala). Horticulturae 7:341–352
Loreto F, Centritto M, Chartzoulakis K (2013) Photosynthetic limitations in olive cultivars with different sensitivity to salt stress. Plant Cell Environ 26:595–601
Nasser AW, El Sheekh MM, Zeineldein MH, Al Maghraby D, Hassan IA (2022) Physiological, morphological, and growth effects of microplastics on freshwater alga Chlorella vulgaris. Rend Fis Acc Lincei 33:815–821. https://doi.org/10.1007/s12210-022-01104-6
Oi T, Enomoto S, Nakao T, Arai S, Yamane K, Taniguchi M (2020) Three-dimensional ultrastructural change of chloroplasts in rice mesophyll cells responding to salt stress. Ann Bot 125:833–840
Rahman A, Hossain MS, Mahmud JA, Nahar K, Hasanuzzaman M, Fujita M (2016) Manganese-induced salt stress tolerance in rice seedlings: Regulation of ion homeostasis, antioxidant defense and glyoxalase systems. Physiol Mol Biol Plants 22:291–306
Santos CV (2004) Regulation of chlorophyll biosynthesis and degradation by salt stress in sunflower leaves. Sci Hortic 103:93–99
Srivastava V, Anish SA, Kumari SK (2015) Analysis on effect of cold stress in bean seeds (Phaseolus vulgaris l). Amer J Biosci 3:145–166
Taffouo VD, Wamba OF, Yombi E, Nono GV, Akoa A (2010) Growth, yield, water status and ionic distribution response of three bambara groundnut (Vigna subterranean L. verdc.) landraces grown under saline conditions. Int J Botany 6:53–58
Taia W, Basahi J, Hassan IA (2013) Impact of ambient air on physiology, pollen tube growth and pollen germination in pepper (Capsicum annuum L.). Pak J Bot 45:921–926
Tiwari BS, Bose A, Ghosh B (2008) Photosynthesis in rice under a salt stress. Photosynthetica 34:303–306
Torche Y, Blair M, Saida C (2018) Biochemical, physiological and phenological genetic analysis in common bean (Phaseolus vulgaris L.) under salt stress. Annals of Agric Sci 63:153–161
Wilkinson S, Clephan AL, Davies WG (2010) Rapid low temperature-induced stomatal closure occurs in cold-tolerant Commelina communis leaves but not in cold-sensitive tobacco leaves, via a mechanism that involves apoplastic calcium but not abscisic acid. Plant Physiol 126:1566–1578
Yamane K, Kawasaki M, Taniguchi M, Miyake H (2008) Correlation between chloroplast ultrastructure and chlorophyll fluorescence characteristics in the leaves of rice (Oryza sativa L.) grown under salinity. Plant Prod Sci 1:139–145
Younis ME, Rizwan M, Tourky SMN (2021) Assessment of early physiological and biochemical responses in chia (Salvia hispanica L) sprouts under salt stress. Acta Physiol Plant 43:121
Zeidan MS (2002) Effect of sowing dates and urea foliar application on growth and seed yield of determinate faba bean (Vicia faba L.) under Egyptian conditions. Egypt J Agron 24:93–102
Zhou R, Hyldgaard B, Yu X, Rosenqvist E, Ugarte RM, Yu S, Wu Z, Ottosen C-O, Zhao T (2018) Phenotyping of faba beans (Vicia faba L.) under cold and heat stresses using chlorophyll fluorescence. Euphytica 214:68–73
Acknowledgements
The authors would like to thank the anonymous reviewers for their invaluable comments. We are indebted to Prof Anne McGill (Edinburgh University, UK) for her thorough revision of this manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors. There were no human or animal materials involved in this research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Dakak, R.A., Badr, R.H., Zeineldein, M.H. et al. Effect of chilling and salinity stress on photosynthetic performance and ultrastructure of chloroplast in faba beans (Vicia faba L.) leaves. Rend. Fis. Acc. Lincei 34, 447–456 (2023). https://doi.org/10.1007/s12210-022-01131-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12210-022-01131-3