Abstract
Great attention has been paid to cofacial porphyrins due to their many unique advantages over their monomeric analogs. However, their synthesis is usually complicated. In this work, a facile impregnation method for preparing heterogenized, cofacially stacked porphyrins is proposed. An anionic porphyrin is introduced as an underlayer for immobilization of cationic cobalt porphyrin via electrostatic force. The metal center of the underlying molecule contributes to the electronic structure of the upper cationic cobalt porphyrin. Screening reveals the anionic iron porphyrin to be the most efficient underlayer molecule, lowering the activation energy barrier of CO2 electroreduction, with an improved turnover frequency by 74% to 8.0 s−1 at − 0.6 V versus RHE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metal porphyrins have received great attention as catalysts because of the clear molecular structures, which is beneficial for understanding the structure–activity relationships. Besides, the electronic structure of the catalytically active metal center, coordinated with four nitrogen atoms, could be effectively tuned by the surrounding ligands [1,2,3,4]. So far, there have been a variety of porphyrin-based molecular catalysts reported active for oxygen reduction reaction, oxygen evolution reaction, hydrogen evolution reaction, and CO2 electroreduction (CO2RR) [5,6,7,8,9].
Motivated by natural enzymes with dual metal centers, such as cytochrome c oxidase and carbon monoxide dehydrogenase, much effort has been devoted to designing cofacial systems with two porphyrin units [10,11,12,13,14], probably because the presence of two metal centers can distribute the demands for redox and coordination numbers across two sites to favor reactions involving the multielectron transfer and a cofacial configuration could optimize the transition state energy level and therefore result in a more enhanced activity than their monomeric analogs [15]. In the past decade, the synthesis of numerous cofacial porphyrin catalysts has been reported for different reactions [16,17,18,19,20,21]. Taking the CO2RR, an essential reaction to mitigate greenhouse CO2 emissions, as an example [22, 23]. Mohamed et al. [24] reported a cofacial iron tetraphenyl porphyrin (FeTPP) dimer for electrochemical CO2-to-CO reduction in DMF/H2O solution, achieving high Faradaic efficiency (FE) of 95% and outstanding turnover frequency (TOF) number of 4300 s−1 at a moderate overpotential of 660 mV. They further assembled the cofacial molecule onto a fluorine-doped tin oxide electrode with a phosphonic acid anchor, showing high activity and stability for CO2RR in aqueous electrolytes [10]. Zahran et al. [25] demonstrated that the substituents on cofacial porphyrin rings greatly affected the catalysis process, and electron-donating groups such as mesityl could lead to increased TOF values. The concept of cofacial porphyrins has also been extended to fabricating the porous organic cage composed of six iron porphyrin units. The supramolecular catalyst produced CO with near-quantitative Faradaic efficiency, with activities reaching 55250 turnovers [26].
However, the synthesis of cofacial porphyrins usually involves a series of complicated procedures preventing their large-scale production. Herein, the neutral tetraphenyl porphyrin molecules are functionalized with cationic methylanilinium (–N(CH3)3+) and anionic sulfonate groups (–SO3−) (Scheme 1), with the electrostatic interaction between these two substituents driving the self-assembly of the cofacial porphyrin system. Characterization measurements, electrochemical analysis, and density functional theory (DFT) calculations reveal a strong electronic interaction between the metal centers, significantly decreasing the reaction barrier and resulting in a more improved CO2RR performance.
Experimental
Catalyst Synthesis
Reduced graphene oxide (rGO, surface area 500 m2/g) was purchased from Tokyo Chemical Industry Co., Ltd. and used as received. Cobalt tetra-(4-N,N,N-trimethylanilinium) porphyrin (CoTMAP), cobalt tetrasulfonatophenyl porphyrin tetrasodium (CoTSPP), iron tetrasulfonatophenyl porphyrin tetrasodium (FeTSPP), and nickel tetrasulfonatophenyl porphyrin tetrasodium (NiTSPP) were synthesized using a reported procedure [27]. Cofacial porphyrins supported on rGO were prepared by two-step incipient wetness impregnation (Fig. 1a). Briefly, CoTSPP (1.5 mg) was dissolved in deionized water and impregnated on rGO (36.5 mg). The mixture was aged for 12 h followed by vacuum drying at 80 ℃ overnight. A second impregnation step was then conducted to further impregnate CoTMAP (2 mg). Again, a 12-h aging step was performed to allow a sufficient interaction between the CoTMAP and CoTSPP molecules. The resulting catalyst, denoted as Co/Co-Por, was eventually dried in a vacuum oven at 80 ℃ overnight. The same procedures were also done to prepare the Ni/Co-Por and Fe/Co-Por catalysts, but using NiTSPP and FeTSPP, respectively, in the first impregnation step. Inductively coupled plasma-atomic emission spectroscopy (ICP-OES) was carried out to calculate the actual Co contents in Co/Co-Por, Ni/Co-Por, and Fe/Co-Por of 0.51%, 0.24%, and 0.26%, respectively, which are close to their nominal values.
Catalyst Characterizations
ICP measurements were conducted using an Agilent 725 ICP-OES. Catalyst morphology was analyzed using a JEOL JEM-2100F field emission source transmission electron microscope (TEM) operating at 200 kV. X-ray photoelectron spectroscopy (XPS) analysis was obtained using a Thermo ESCALAB 250Xi spectrometer equipped with a monochromatic AlK radiation source (1486.6 eV, pass energy 20.0 eV). The diffuse reflectance UV–Vis spectrum was recorded in the 200–800 nm range using a UV–Vis spectrophotometer (Lambda 750, PerkinElmer, Inc.).
Electrochemical Measurements
To prepare the working electrode, the catalyst powder (5 mg) and Nafion solution (13 μL, 5 wt%, Sigma-Aldrich) were dispersed in ethanol (1 mL, Aladdin, ACS, ≥ 99.5%) and sonicated for 1 h to form a uniform catalyst ink. Then, the ink was drop cast onto carbon paper (Toray, TGP-H-060) to reach a catalyst loading of 0.1 mg/cm2. Its electrochemical performance in CO2 reduction was tested in a customized H-cell separated by a Nafion 117 membrane. Platinum foil and a leak-free Ag/AgCl electrode (LF-2, Innovative Instrument Inc.) were used as the counter and reference electrodes, respectively. Before the experiments, sodium bicarbonate (1.75 mL, 0.5 M, Sigma-Aldrich, ≥ 99.5%) solution was injected into the working and counter compartments. CO2 was purged into the chambers for 10 min before electrochemical polarization. Electrochemical responses were recorded by a CHI650E potentiostat. Electrolyte resistance between the reference and working electrodes was measured by potential electrochemical impedance spectroscopy and manually compensated. During the electrolysis, gas products were analyzed using an online gas chromatograph equipped with a Hayesep-D column, TCD detector, and FID detector.
The Faradaic efficiencies of gas products were calculated using the following equation:
where i represents CO or H2; x is the concentration of CO or H2; ν is the feeding rate of CO2; n is the electron transfer number (2, for both CO and H2); F is the Faradaic constant (96,485 C/mol); and I is the average current during electrolysis.
Theoretical Calculation
DFT calculations were performed using the Gaussian09 at the PBE0 level using the LanL2DZ basis set [28,29,30,31] for metal atoms (Fe, Co, and Ni) and 6-31G(d) basis set for non-metal atoms (H, N, C, O, and S).
Results and Discussion
Incipient wetness impregnation can allow anionic porphyrins to uniformly immobilize onto the rGO support through π–π interactions and sufficient interaction between the cationic porphyrins and anionic ones through electrostatic force. Figure 1b shows the TEM image of the as-prepared Co/Co-Por catalyst with a clean two-dimensional morphology. The energy-dispersive X-ray spectroscopy (EDS) maps also show the uniform distribution of C, N, and Co and no apparent aggregates. To verify, we also prepared the hybrid catalyst at a high loading by impregnating 15 mg of CoTSPP on 35 mg of rGO. The resulting catalyst shows many porphyrin aggregates with an estimated average size of 5–10 nm (Fig. S1). From this perspective, a low porphyrin loading, which requires a dilute porphyrin-water solution for impregnation, is key to the successful preparation of the highly dispersed hybrid catalysts.
XPS and UV–Vis spectroscopy were performed to study the electronic structure of Co/Co-Por. Figure 1c shows the XPS Co 2p spectrum showing Co 2p3/2 peaks at 781.7 and 780.6 eV for CoTMAP and CoTSPP, respectively. The different binding energies of the Co 2p3/2 band are due to the distinct electronegativity features of the peripheral functionalities. The electron-withdrawing methylanilinium group decreases the electron density around the Co center, while the electron-donating sulfonate group increases the electron density around cobalt [27]. The two-porphyrin cofacial Co/Co-Por reveals a neutralized binding energy value of 781.3 eV for the Co 2p3/2 signal, indicating the electrons within the stacked molecule system to be significantly redistributed, as confirmed by the shifted Soret band for Co/Co-Por (419.8 nm) as compared to CoTMAP (426.3 nm) or CoTSPP (413.4 nm) (Fig. 1d). The Q bands for all three samples could hardly be distinguished from the strong background of rGO absorbance. The above results reveal the intimate contact between the CoTMAP and CoTSPP molecules.
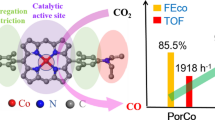
Ni/Co-Por and Fe/Co-Por were also prepared by introducing NiTSPP and FeTSPP as the sublayer porphyrins, respectively, for systematically investigating the structure–activity relationship of cofacial porphyrins during the electrochemical CO2 reduction. For comparison, monomeric CoTMAP, CoTSPP, FeTSPP, and NiTSPP catalysts on rGO were also prepared at a content of 5 wt%. The EDS maps in Fig. S2 confirm the highly disperse NiTSPP and CoTMAP in Ni/Co-Por and uniform distribution of FeTSPP and CoTMAP in Fe/Co-Por. In addition, the binding energies of the Co 2p3/2 signal are also found to shift in these two catalysts due to the electronic interactions (Fig. S3). The steady-state electroreduction of CO2 was conducted in a customized H-cell in a CO2-saturated 0.5 M NaHCO3 electrolyte. Cyclic voltammetry measurements show prominent reduction currents at potentials more negative than approximately − 0.4 V versus RHE (Fig. S4). CoTMAP exhibits a stable current at a potential of − 0.6 V versus RHE with an average current density of 4.6 mA/cm2 during 1 h of electrolysis (Fig. 2a, Fig. S5). Moreover, the activities for cofacial porphyrins follow the order Fe/Co-Por (8.0 mA/cm2) > Co/Co-Por (6.5 mA/cm2) > Ni/Co-Por (5.7 mA/cm2). Current densities are much lower in the sulfonate group functionalized metal porphyrin than in the methylanilinium group functionalized CoTMAP catalyst. CoTSPP, NiTSPP, and FeTSPP have overall current densities of 1.4, 0.7, and 1.1 mA/cm2, respectively, at − 0.6 V versus RHE (Fig. S6), in agreement with our previous study that CO2 reduction favors cationic substituents over neutral or anionic ones [27]. Excellent selectivities toward CO production are shown by all tested catalysts, with FECO values over 90% (Fig. 2b, Fig. S7).
a Overall current densities and b Faradaic efficiencies of CO and H2 over CoTMAP and cofacial porphyrins. c The partial current density of CO (jCO) over cofacial porphyrin containing CoTSPP and H2TMAP with different weight ratios. d Calculated TOFCO values for CoTMAP and cofacial porphyrin catalysts (reaction was performed at − 0.6 V versus RHE in a CO2-saturated 0.5 M NaHCO3 electrolyte)
The resistances of the catalysts were investigated by AC impedance analysis at an open-circuit voltage (OCV). The Nyquist plots demonstrate that the four samples have similar interfacial charge transfer resistance (Rct), possibly due to the identical and low catalyst loading. TOF values were calculated for investigating the intrinsic reactivity of each catalyst based on the number of CoTMAP molecules on the top layer. It could be argued that the underlying MTSPP may not be completely stacked (i.e., covered) and may still contribute to the reductive currents. Therefore, a set of H/Co-Por samples were prepared as a control with various H2TMAP loadings for the second impregnation step. As described above, CoTSPP alone delivers a jCO of 1.3 mA/cm2 (Fig. 2c). The introduction of H2TMAP, which is inactive for CO production, suppresses the current densities. This serves as direct proof that the cationic porphyrins preferentially graft on the pre-deposited anionic porphyrins by electrostatic force and block the underlying metal centers from accessing the reactants. The partial current density of CO becomes negligible (< 10% of the original value) with weight ratios of H2TMAP/CoTSPP over 1.3, implying the complete assembly of H/Co-Por cofacial porphyrin. The control experiment also validates our calculation protocol of TOF values for the samples discussed here. The CoTMAP catalyst exhibits a TOFCO of 4.4 s−1 (Fig. 2d), similar to that of Ref. [27]. Fabricating the cofacial porphyrins using MTSPP leads to improved intrinsic activities with the order of Fe/Co-Por (7.6 s−1) > Co/Co-Por (6.1 s−1) > Ni/Co-Por (5.4 s−1).
DFT calculations were performed for analyzing the thermochemical reaction energetics of CO2RR on CoTMAP, Co/Co-Por, Ni/Co-Por, and Fe/Co-Por. The molecular structures were first optimized for the cofacial porphyrins according to the H-aggregation configuration. The introduced H2TMAP blocked the underlying metal sites (Fig. 2c), which implies a high overlapping degree (Fig. S8). CO2-to-CO electroreduction by molecular catalysts involves *COOH and *CO intermediates [32,33,34]. *COOH formation is usually considered the rate-determining step (RDS), and the *CO species are believed to desorb promptly to form the CO products. CoTMAP, Co/Co-Por, Ni/Co-Por, and Fe/Co-Por have Tafel slopes of 114.9, 123.8, 118.4, and 133.7 mV/dec (Fig. S9). Furthermore, a CO2 reaction order of approximately 1 is determined for all the catalysts, indicating the CO2-to-*COOH transformation to be the rate-limiting step (Fig. S10)[9]. *COOH formation is the most energy-demanding, as suggested by the DFT results, associated with the highest energy barrier of + 0.6 eV compared to other reaction steps (Fig. 3a). Figure 3b shows the free energy diagrams for all CoTMAP-based catalysts. While all the cofacial porphyrins show the same RDS for CO2-to-CO conversion as the monomeric CoTMAP, their reaction energy barriers appear to be lower (Fig. S11). Then, the calculated theoretical reaction overpotentials over the tested catalysts follow the order Fe/Co-Por (0.58 V) < Co/Co-Por (0.60 V) < Ni/Co-Por (0.65 V) < CoTMAP (0.68 V). The above results show that, in addition to peripheral substituents, metal atoms from the underlying catalytically inactive porphyrin molecules also play an important role in tuning the electronic structure of the catalytically active porphyrin molecules on the top layer. Correspondingly, the log(TOF) values appear to be linearly correlated with the theoretical overpotential values.
Conclusions
In summary, a two-step impregnation method is proposed to prepare cofacially stacked porphyrins by utilizing the electrostatic force between the cationic and anionic porphyrin molecules, which not only avoids the common tedious chemical synthesis route but also effectively tunes the electronic structure of the molecular catalysts, eventually leading to a lower reaction energy barrier and a significantly improved reaction rate for CO2 electroreduction. Our method should theoretically be adaptable to other carbonaceous supports such as carbon black and carbon nanotubes. In addition, the facile approach has the potential to guide the rational design of other cofacial porphyrin catalysts.
References
Manbeck GF, Fujita E (2015) A review of iron and cobalt porphyrins, phthalocyanines and related complexes for electrochemical and photochemical reduction of carbon dioxide. J Porphyrins Phthalocyanines 19(1–3):45–64
Sun CF, Gobetto R, Nervi C (2016) Recent advances in catalytic CO2 reduction by organometal complexes anchored on modified electrodes. New J Chem 40(7):5656–5661
Elouarzaki K, Kannan V, Jose V et al (2019) Recent trends, benchmarking, and challenges of electrochemical reduction of CO2 by molecular catalysts. Adv Energy Mater 9(24):1900090
Birdja YY, Pérez-Gallent E, Figueiredo MC et al (2019) Advances and challenges in understanding the electrocatalytic conversion of carbon dioxide to fuels. Nat Energy 4(9):732–745
Choi J, Kim J, Wagner P et al (2019) Energy efficient electrochemical reduction of CO2 to CO using a three-dimensional porphyrin/graphene hydrogel. Energy Environ Sci 2:747–755
Hu XM, Rønne MH, Pedersen SU et al (2017) Enhanced catalytic activity of cobalt porphyrin in CO2 electroreduction upon immobilization on carbon materials. Angew Chem Int Ed 56(23):6468–6472
Tatin A, Comminges C, Kokoh B et al (2016) Efficient electrolyzer for CO2 splitting in neutral water using earth-abundant materials. Proc Natl Acad Sci 113(20):5526–5529
Zion N, Friedman A, Levy N et al (2018) Bioinspired electrocatalysis of oxygen reduction reaction in fuel cells using molecular catalysts. Adv Mater 30(41):1800406
Zhu M, Chen J, Huang L et al (2019) Covalently grafting cobalt porphyrin onto carbon nanotubes for efficient CO2 electroreduction. Angew Chem Int Ed Engl 58(20):6595–6599
Mohamed EA, Zahran ZN, Naruta Y (2017) Efficient heterogeneous CO2 to CO conversion with a phosphonic acid fabricated cofacial iron porphyrin dimer. Chem Mater 29(17):7140–7150
Guilard R, Brandes S, Tardieux C et al (1995) Synthesis and characterization of cofacial metallodiporphyrins involving cobalt and Lewis acid metals: new dinuclear multielectron redox catalysts of dioxygen reduction. J Am Chem Soc 117(47):11721–11729
Peljo P, Murtomäki L, Kallio T et al (2012) Biomimetic oxygen reduction by cofacial porphyrins at a liquid-liquid interface. J Am Chem Soc 134(13):5974–5984
Takai A, Gros CP, Barbe JM et al (2009) Enhanced electron-transfer properties of cofacial porphyrin dimers through π–π interactions. Chemistry 15(13):3110–3122
Collman JP, Kim K, Leidner CR (1987) Synthesis, characterization, and electrochemistry of novel diruthenium cofacial porphyrin dimers. Inorg Chem 26(7):1152–1157
Oldacre AN, Friedman AE, Cook TR (2017) A self-assembled cofacial cobalt porphyrin prism for oxygen reduction catalysis. J Am Chem Soc 139(4):1424–1427
Collman JP, Denisevich P, Konai Y et al (1980) Electrode catalysis of the four-electron reduction of oxygen to water by dicobalt face-to-face porphyrins. J Am Chem Soc 102(19):6027–6036
Collman JP, Bencosme CS, Durand RR Jr et al (1983) Mixed-metal face-to-face porphyrin dimers. J Am Chem Soc 105(9):2699–2703
Durand RR, Bencosme CS, Collman JP et al (1983) Mechanistic aspects of the catalytic reduction of dioxygen by cofacial metalloporphyrins. J Am Chem Soc 105(9):2710–2718
Nakamura T, Ube H, Shionoya M (2013) Silver-mediated formation of a cofacial porphyrin dimer with the ability to intercalate aromatic molecules. Angew Chem Int Ed Engl 52(46):12096–12100
Oliveri CG, Heo J, Nguyen ST et al (2007) A convergent coordination chemistry-based approach to dissymmetric macrocyclic cofacial porphyrin complexes. Inorg Chem 46(19):7716–7718
Suijkerbuijk BMJM, Tooke DM, Spek AL et al (2007) One-dimensional, cofacial porphyrin polymers formed by self-assembly of meso-tetrakis(ERE donor) zinc(II) porphyrins. Chem Asian J 2(7):889–903
Hepburn C, Adlen E, Beddington J et al (2019) The technological and economic prospects for CO2 utilization and removal. Nature 575(7781):87–97
Gruber N, Clement D, Carter BR et al (2019) The oceanic sink for anthropogenic CO2 from 1994 to 2007. Science 363(6432):1193–1199
Mohamed EA, Zahran ZN, Naruta Y (2015) Efficient electrocatalytic CO2 reduction with a molecular cofacial iron porphyrin dimer. Chem Commun Camb Engl 51(95):16900–16903
Zahran ZN, Mohamed EA, Naruta Y (2016) Bio-inspired cofacial Fe porphyrin dimers for efficient electrocatalytic CO2 to CO conversion: overpotential tuning by substituents at the porphyrin rings. Sci Rep 6:24533
Smith PT, Benke BP, Cao Z et al (2018) Iron porphyrins embedded into a supramolecular porous organic cage for electrochemical CO2 reduction in water. Angew Chem 130(31):9832–9836
Zhu MH, Yang DT, Ye RQ et al (2019) Inductive and electrostatic effects on cobalt porphyrins for heterogeneous electrocatalytic carbon dioxide reduction. Catal Sci Technol 9(4):974–980
Adamo C, Barone V (1999) Toward reliable density functional methods without adjustable parameters: the PBE0 model. J Chem Phys 110(13):6158–6170
Hay PJ, Wadt WR (1985) Ab initio effective core potentials for molecular calculations: potentials for the transition metal atoms Sc to Hg. J Chem Phys 82(1):270–283
Wadt WR, Hay PJ (1985) Ab initio effective core potentials for molecular calculations: potentials for main group elements Na to Bi. J Chem Phys 82(1):284–298
Hay PJ, Wadt WR (1985) Ab initio effective core potentials for molecular calculations: potentials for K to Au including the outermost core orbitals. J Chem Phys 82(1):299–310
Lin S, Diercks CS, Zhang YB et al (2015) Covalent organic frameworks comprising cobalt porphyrins for catalytic CO2 reduction in water. Science 349(6253):1208–1213
Costentin C, Drouet S, Robert M et al (2012) A local proton source enhances CO2 electroreduction to CO by a molecular Fe catalyst. Science 338(6103):90–94
Zhu MH, Ye RQ, Jin K et al (2018) Elucidating the reactivity and mechanism of CO2 electroreduction at highly dispersed cobalt phthalocyanine. ACS Energy Lett 3(6):1381–1386
Acknowledgements
This work was sponsored by the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning and Shanghai Sailing Program (No. 19YF1410600). R.Y. acknowledge the Young Scientists Fund of the National Natural Science Foundation of China (No. 21905240) and the State Key Laboratory of Marine Pollution (SKLMP) Seed Collaborative Research Fund.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tian, P., Su, J., Song, Y. et al. Electroreduction of Carbon Dioxide by Heterogenized Cofacial Porphyrins. Trans. Tianjin Univ. 28, 73–79 (2022). https://doi.org/10.1007/s12209-021-00305-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12209-021-00305-8