Abstract
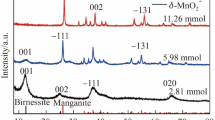
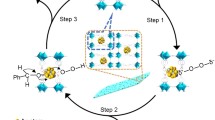
Anisotropic MnO2 nanostructures, including α-phase nanowire, α-phase nanorod, δ-phase nanosheet, α + δ-phase nanowire, and amorphous floccule, were synthesized by a simple hydrothermal method through adjusting the pH of the precursor solution and using different counterions. The catalytic properties of the as-synthesized MnO2 nanomaterials in the selective oxidation of benzyl alcohol (BA) and 5-hydroxymethylfurfural (HMF) were evaluated. The effects of micromorphology, phase structure, and redox state on the catalytic activity of MnO2 nanomaterials were investigated. The results showed that the intrinsic catalytic oxidation activity was mainly influenced by the unique anisotropic structure and surface chemical property of MnO2. With one-dimensional and 2D structures exposing highly active surfaces, unique crystal forms, and high oxidation state of Mn, the intrinsic activities for MnO2 catalysts synthesized in pH 1, 5, and 10 solutions (denoted as MnO2-pH1, MnO2-pH5, and MnO2-pH10, respectively) were twice higher than those of other MnO2 catalysts in oxidation of BA and HMF. With a moderate aspect ratio, the α + δ nanowire of MnO2-pH10 exhibited the highest average oxidation state, most abundant active sites, and the best catalytic oxidation activity.






Similar content being viewed by others
References
Zhou KB, Li YD (2012) Catalysis based on nanocrystals with well-defined facets. Angew Chem Int Ed 51(3):602–613
Li Y, Yang XY, Feng Y et al (2012) One-dimensional metal oxide nanotubes, nanowires, nanoribbons, and nanorods: synthesis, characterizations, properties and applications. Crit Rev Solid State Mater Sci 37(1):1–74
Zhang Q, Wang HY, Jia XL et al (2013) One-dimensional metal oxide nanostructures for heterogeneous catalysis. Nanoscale 5(16):7175–7183
Yang HG, Sun CH, Qiao SZ et al (2008) Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 453(7195):638–641
Tian N, Zhou ZY, Sun SG et al (2007) Synthesis of tetrahexahedral platinum nanocrystals with high-index facets and high electro-oxidation activity. Science 316(5825):732–735
Mostafa S, Behafarid F, Croy JR et al (2010) Shape-dependent catalytic properties of Pt nanoparticles. J Am Chem Soc 132(44):15714–15719
Browne MP, Sofer Z, Pumera M (2019) Layered and two dimensional metal oxides for electrochemical energy conversion. Energy Environ Sci 12(1):41–58
Xu HM, Yan NQ, Qu Z et al (2017) Gaseous heterogeneous catalytic reactions over Mn-based oxides for environmental applications: a critical review. Environ Sci Technol 51(16):8879–8892
Zheng XH, Li YL, Zhang LY et al (2019) Insight into the effect of morphology on catalytic performance of porous CeO2 nanocrystals for H2S selective oxidation. Appl Catal B Environ 252:98–110
Chen M, Nikles DE (2002) Synthesis, self-assembly, and magnetic properties of FexCoyPt100-x-y Nanoparticles. Nano Lett 2(3):211–214
Bliznyuk V, Singamaneni S, Sahoo S et al (2009) Self-assembly of magnetic Ni nanoparticles into 1D arrays with antiferromagnetic order. Nanotechnology 20(10):105606
Bao NZ, Shen LM, Padhan P et al (2008) Self-assembly and magnetic properties of shape-controlled monodisperse CoFe2O4 nanocrystals. Appl Phys Lett 92(17):173101
Zhang K, Han XP, Hu Z et al (2015) Nanostructured Mn-based oxides for electrochemical energy storage and conversion. Chem Soc Rev 44(3):699–728
Yao WT, Odegard GM, Huang ZN et al (2018) Cations controlled growth of β-MnO2 crystals with tunable facets for electrochemical energy storage. Nano Energy 48:301–311
Bai BY, Li JH, Hao JM (2015) 1D-MnO2, 2D-MnO2 and 3D-MnO2 for low-temperature oxidation of ethanol. Appl Catal B Environ 164:241–250
Hayashi E, Yamaguchi Y, Kamata K et al (2019) Effect of MnO2 crystal structure on aerobic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid. J Am Chem Soc 141(2):890–900
Miao L, Wang JL, Zhang PY (2019) Review on manganese dioxide for catalytic oxidation of airborne formaldehyde. Appl Surf Sci 466:441–453
Ma JP, Du ZT, Xu J et al (2011) Efficient aerobic oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran, and synthesis of a fluorescent material. Chemsuschem 4(1):51–54
Xiang TF, Liu XM, Yi P et al (2013) Schiff base polymers derived from 2,5-diformylfuran. Polym Int 62(10):1517–1523
Ma JP, Yu WQ, Wang M et al (2013) Advances in selective catalytic transformation of ployols to value-added chemicals. Chin J Catal 34(3):492–507
Yang ZZ, Deng J, Pan T et al (2012) A one-pot approach for conversion of fructose to 2,5-diformylfuran by combination of Fe3O4-SBA-SO3H and K-OMS-2. Green Chem 14(11):2986
Pal P, Saravanamurugan S (2019) Recent advances in the development of 5-hydroxymethylfurfural oxidation with base (nonprecious)-metal-containing catalysts. Chemsuschem 12(1):145–163
Zhang ZH, Huber GW (2018) Catalytic oxidation of carbohydrates into organic acids and furan chemicals. Chem Soc Rev 47(4):1351–1390
Enache DI, Edwards JK, Landon P et al (2006) Solvent-free oxidation of primary alcohols to aldehydes using Au-Pd/TiO2 catalysts. Science 311(5759):362–365
Nie JF, Liu HC (2014) Efficient aerobic oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran on manganese oxide catalysts. J Catal 316:57–66
Ke QP, Jin YX, Ruan F et al (2019) Boosting the activity of catalytic oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran over nitrogen-doped manganese oxide catalysts. Green Chem 21(16):4313–4318
Liu P, Duan JH, Ye Q et al (2018) Promoting effect of unreducible metal doping on OMS-2 catalysts for gas-phase selective oxidation of ethanol. J Catal 367:115–125
Son YC, Makwana VD, Howell AR et al (2001) Efficient, catalytic, aerobic oxidation of alcohols with octahedral molecular sieves. Angew Chem Int Ed 40(22):4280–4283
Perner A, Holl K, Ilic D et al (2002) A new MnOx cathode material for rechargeable Lithium batteries. Eur J Inorg Chem 2002(5):1108–1114
Chitrakar R, Kanoh H, Kim YS et al (2001) Synthesis of layered-type hydrous manganese oxides from monoclinic-type LiMnO2. J Solid State Chem 160(1):69–76
Golden DC, Chen CC, Dixon JB (1986) Synthesis of todorokite. Science 231(4739):717–719
Shen YF, Zerger RP, DeGuzman RN et al (1993) Manganese oxide octahedral molecular sieves: preparation, characterization, and applications. Science 260(5107):511–515
Luo J, Suib SL (1997) Preparative parameters, magnesium effects, and anion effects in thee crystallization of birnessites. J Phys Chem B 101:10403–10413
Ching S, Roark JL, Suib SL et al (1997) Sol-gel route to the tunneled manganese oxide cryptomelane. Chem Mater 9:750–754
Ching S, Petrovay DJ, Jorgensen ML et al (1997) Sol–gel synthesis of layered birnessite-type manganese oxides. Inorg Chem 36(5):883–890
Zhang N, Cheng FY, Liu JX et al (2017) Rechargeable aqueous zinc–manganese dioxide batteries with high energy and power densities. Nat Commun 8:405
Najafpour MM, Renger G, Hołyńska M et al (2016) Manganese compounds as water-oxidizing catalysts: from the natural water-oxidizing complex to nanosized manganese oxide structures. Chem Rev 116(5):2886–2936
Shi FJ, Wang F, Dai HX et al (2012) Rod-, flower-, and dumbbell-like MnO2: highly active catalysts for the combustion of toluene. Appl Catal A Gen 433–434:206–213
Li JM, Qu ZP, Qin Y et al (2016) Effect of MnO2 morphology on the catalytic oxidation of toluene over Ag/MnO2 catalysts. Appl Surf Sci 385:234–240
Tang QW, Jiang LH, Liu J et al (2014) Effect of surface manganese valence of manganese oxides on the activity of the oxygen reduction reaction in alkaline media. ACS Catal 4(2):457–463
Kakizaki H, Ooka H, Hayashi T et al (2018) Evidence that crystal facet orientation dictates oxygen evolution intermediates on rutile manganese oxide. Adv Funct Mater 28(24):1706319
Xu KB, Lin XX, Wang XF et al (2018) Generating more Mn4+ ions on surface of nonstoichiometric MnO2 nanorods via microwave heating for improved oxygen electroreduction. Appl Surf Sci 459:782–787
Wang H, Chen H, Wang Y et al (2019) Performance and mechanism comparison of manganese oxides at different valence states for catalytic oxidation of NO. Chem Eng J 361:1161–1172
Wang X, Li YD (2003) Rare-earth-compound nanowires, nanotubes, and fullerene-like nanoparticles: synthesis, characterization, and properties. Chem Eur J 9(22):5627–5635
DeGuzman RN, Shen YF, Neth EJ et al (1994) Synthesis and characterization of octahedral molecular sieves (OMS-2) having the hollandite structure. Chem Mater 6(6):815–821
Wang X, Li YD (2003) Synthesis and formation mechanism of manganese dioxide nanowires/nanorods. Chem A Eur J 9(1):300–306
Wang AQ, Wang H, Deng H et al (2019) Controllable synthesis of mesoporous manganese oxide microsphere efficient for photo-Fenton-like removal of fluoroquinolone antibiotics. Appl Catal B Environ 248:298–308
Mo SP, Zhang Q, Li JQ et al (2020) Highly efficient mesoporous MnO2 catalysts for the total toluene oxidation: oxygen-vacancy defect engineering and involved intermediates using in situ DRIFTS. Appl Catal B Environ 264:118464
Lee SJ, Gavriilidis A, Pankhurst QA et al (2001) Effect of drying conditions of Au–Mn Co-precipitates for low-temperature CO oxidation. J Catal 200(2):298–308
Xie XF, Gao L (2007) Characterization of a manganese dioxide/carbon nanotube composite fabricated using an in situ coating method. Carbon 45(12):2365–2373
Feng XM, Yan ZZ, Chen NN et al (2013) The synthesis of shape-controlled MnO2/graphene composites via a facile one-step hydrothermal method and their application in supercapacitors. J Mater Chem A 1(41):12818
Han XW, Li CQ, Liu XH et al (2017) Selective oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid over MnOx–CeO2 composite catalysts. Green Chem 19(4):996–1004
Liu H, Cao XJ, Wei JN et al (2019) Efficient aerobic oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran over Fe2O3-promoted MnO2 catalyst. ACS Sustain Chem Eng 7(8):7812–7822
Xu R, Wang X, Wang DS et al (2006) Surface structure effects in nanocrystal MnO2 and Ag/MnO2 catalytic oxidation of CO. J Catal 237(2):426–430
Zahoor A, Jang HS, Jeong JS et al (2014) A comparative study of nanostructured α and δ MnO2 for Lithium oxygen battery application. RSC Adv 4(18):8973
Liang SH, Teng F, Bulgan G et al (2008) Effect of phase structure of MnO2 nanorod catalyst on the activity for CO oxidation. J Phys Chem C 112(14):5307–5315
Acknowledgements
We thank the National Natural Science Foundation of China (No. 21503187) and the “Light of West China” Program of the Chinese Academy of Sciences for the financial support. We also thank Prof. Haichao Liu (College of Chemistry and Molecular Engineering, Peking University, China) for his helpful suggestions and guidance during catalyst characterization.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, H., Song, Y., Liu, X. et al. Preparation of Anisotropic MnO2 Nanocatalysts for Selective Oxidation of Benzyl Alcohol and 5-Hydroxymethylfurfural. Trans. Tianjin Univ. 26, 382–390 (2020). https://doi.org/10.1007/s12209-020-00261-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12209-020-00261-9