Abstract
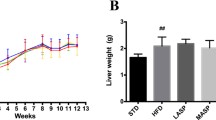
Biochanin A (BCA) and CPe-III peptide, which both exist in chickpea (Cicer arietinum L.), possess significant antihyperlipidemic properties. However, the actual mechanisms of those compounds in inhibiting the dysregulation of lipid metabolism and complicated inflammation have not been well characterized. This study investigated the effects of BCA, CPe-III peptide, and combined BCA and CPe-III peptide (BC) on the expression of genes involved in hepatic lipid and inflammation metabolism. Results demonstrated that BCA, CPe-III peptide, and BC significantly attenuated hepatitis and hyperlipidemia by downregulating those genes involved in pro-inflammatory cytokines (TNF-α), hepatic fatty acid (FA) synthesis (ACC1 and FAS), cholesterol metabolism (SREBP2, HMGCR, and PCSK9), and upregulating key regulators involved in FA oxidation (PPARα and FABP1), lipolysis (ATGL), LDLR, reverse cholesterol transport (ABCA1, SR-B1, and LXRα), and cholesterol catabolism (CYP7A1). Moreover, they also altered the expression of lipid metabolism-related proteins, including SREBP2, PCSK9, LDLR, ABCA1, and CYP7A1. Finally, these results revealed that the combination treatment of BCA and CPe-III peptide resulted in greater antihyperlipidemic activity compared with individual compounds.





Similar content being viewed by others
References
De Sereday MS, Gonzalez C, Giorgini D et al (2004) Prevalence of diabetes, obesity, hypertension and hyperlipidemia in the central area of Argentina. Diabetes Metab 30(4):335–339
Kwon EY, Jung UJ, Park T et al (2015) Luteolin attenuates hepatic steatosis and insulin resistance through the interplay between the liver and adipose tissue in mice with diet-induced obesity. Diabetes 64(5):1658–1669
Maggo SDS, Kennedy MA, Clark DWJ (2011) Clinical implications of pharmacogenetic variation on the effects of statins. Drug Saf 34(1):1–19
Wang KL, Liu CJ, Chao TF et al (2012) Statins, risk of diabetes, and implications on outcomes in the general population. J Am Coll Cardiol 60(14):1231–1238
Afman L, Müller M (2006) Nutrigenomics: from molecular nutrition to prevention of disease. J Am Diet Assoc 106(4):569–576
Mao J, DeMayo FJ, Li HG et al (2006) Liver-specific deletion of acetyl-Coa carboxylase 1 reduces hepatic triglyceride accumulation without affecting glucose homeostasis. Proc Natl Acad Sci USA 103(22):8552–8557
Semenkovich CF (1997) Regulation of fatty acid synthase (FAS). Prog Lipid Res 36(1):43–53
Lu YF, Xu YY, Jin F et al (2014) Icariin is a PPARα activator inducing lipid metabolic gene expression in mice. Molecules 19(11):18179–18191
Muoio DM, Maclean PS, Lang DB et al (2002) Fatty acid homeostasis and induction of lipid regulatory genes in skeletal muscles of peroxisome proliferator-activated receptor (PPAR) α knock-out mice. Evidence for compensatory regulation by PPARα. J Biol Chem 277(29):26089–26097
Feingold KR, Adi S, Staprans I et al (1990) Diet affects the mechanisms by which TNF stimulates hepatic triglyceride production. Am J Physiol 259(2):177–184
Popa C, Netea MG, van Riel PLM et al (2007) The role of TNF-α in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J Lipid Res 48(4):751–762
Chen ZY, Jiao R, Ma KY (2008) Cholesterol-lowering nutraceuticals and functional foods. J Agric Food Chem 56(19):8761–8773
Sato R (2010) Sterol metabolism and SREBP activation. Arch Biochem Biophys 501(2):177–181
Guo YL, Zhang W, Li JJ (2014) PCSK9 and lipid lowering drugs. Clin Chim Acta 437(1):66–71
Chiang JYL (2009) Bile acids: regulation of synthesis. J Lipid Res 50(10):1955–1966
Bonamassa B, Moschetta A (2013) Atherosclerosis: lessons from LXR and the intestine. Trends Endocrinol Metab 24(3):120–128
Khalil A, Berrougui H, Pawelec G et al (2012) Impairment of the ABCA1 and SR-BI-mediated cholesterol efflux pathways and HDL anti-inflammatory activity in Alzheimer’s disease. Mech Ageing Dev 133(1):20–29
Harini R, Sundaresan A, Pugalendi KV (2012) Antihyperlipidemic effect of biochanin A on streptozotocin induced diabetic rats. J Pharm Res 5(1):707–710
Park HS, Hur HJ, Kim SH et al (2016) Biochanin A improves hepatic steatosis and insulin resistance by regulating the hepatic lipid and glucose metabolic pathways in diet-induced obese mice. Mol Nutr Food Res 60(9):1944–1955
Xue ZH, Gao J, Zhang ZJ et al (2012) Antihyperlipidemic and antitumor effects of chickpea albumin hydrolysate. Plant Food Hum Nutr 67(4):393–400
Kou XH, Gao J, Xue ZH et al (2013) Purification and identification of antioxidant peptides from chickpea (Cicer Arietinum L.) albumin hydrolysates. LWT-Food. Sci Technol 50(2):591–598
Miller L (2010) Analyzing gels and western blots with ImageJ. Lukemiller. Org. http://lukemiller.org/index.php/2010/11/analyzing-gels-and-western-blots-with-image-j/. Accessed 1 Mar 2016
Norris GH, Porter CM, Jiang C et al (2017) Dietary sphingomyelin attenuates hepatic steatosis and adipose tissue inflammation in high-fat-diet-induced obese mice. J Nutr Biochem 40:36–43
Zhang RJ, Yu Y, Hu S et al (2016) Sesamin ameliorates hepatic steatosis and inflammation in rats on a high-fat diet via LXRα and PPARα. Nutr Res 36(9):1022–1030
Lira FS, Rosa Neto JC, Antunes BMM et al (2014) The relationship between inflammation, dyslipidemia and physical exercise: from the epidemiological to molecular approach. Curr Diabetes Rev 10(6):391–396
Galli C, Calder PC (2009) Effects of fat and fatty acid intake on inflammatory and immune responses: a critical review. Ann Nutr Metab 55(1–3):123–139
Navab M, Anantharamaiah GM, Fogelman AM (2005) The role of high-density lipoprotein in inflammation. Trends Cardiovas Med 15(4):158–161
Breikaa RM, Algandaby MM, El-Demerdash E et al (2013) Multimechanistic antifibrotic effect of biochanin A in rats: implications of proinflammatory and profibrogenic mediators. PLoS ONE 8(7):e69276
Boden G, She PX, Mozzoli M et al (2006) Free fatty acids produce insulin resistance and activate the proinflammatory nuclear factor-κB pathway in rat liver. Diabetes 54(12):3458–3465
Freigang S, Ampenberger F, Weiss A et al (2013) Fatty acid-induced mitochondrial uncoupling elicits inflammasome- independent IL-1α and sterile vascular inflammation in atherosclerosis. Nat Immunol 14(10):1045–1053
Guzmán C, Benet M, Pisonero-Vaquero S et al (2013) The human liver fatty acid binding protein (FABP1) gene is activated by FOXA1 and PPARα; and repressed by C/EBPα: implications in FABP1 down-regulation in nonalcoholic fatty liver disease. Biochim Biophys Acta 1831(4):803–818
Varga T, Czimmerer Z, Nagy L (2011) PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim Biophys Acta 1812(8):1007–1022
Qiu LX, Ye H, Chen LM et al (2012) Red clover extract ameliorates dyslipidemia in streptozotocin-induced diabetic C57BL/6 mice by activating hepatic PPARα. Phytother Res 26(6):860–864
Mueller M, Hobiger S, Jungbauer A (2010) Red clover extract: a source for substances that activate peroxisome proliferator-activated receptor α and ameliorate the cytokine secretion profile of lipopolysaccharide-stimulated macrophages. Menopause 17(2):379–387
Le NA, Brown WV (2011) Triglyceride-rich lipoproteins. In: Grundy SM (ed) Atlas of atherosclerosis and metabolic syndrome. Springer, New York, USA, pp 59–91
Perona JS, Covas MI, Fitó M et al (2010) Reduction in systemic and VLDL triacylglycerol concentration after a 3-month Mediterranean-style diet in high-cardiovascular-risk subjects. J Nutr Biochem 21(9):892–898
Yang JH, Bang MA, Jang CH et al (2015) Alginate oligosaccharide enhances LDL uptake via regulation of LDLR and PCSK9 expression. J Nutr Biochem 26(11):1393–1400
Horton JD, Shah NA, Warrington JA et al (2003) Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc Natl Acad Sci USA 100(21):12027–12032
Maxwell KN, Soccio RE, Duncan EM et al (2003) Novel putative SREBP and LXR target genes identified by microarray analysis in liver of cholesterol-fed mice. J Lipid Res 44(11):2109–2119
Liu Q, Guo Y, Gui YJ et al (2016) Is sEHi lowering LDL-C by reducing expression of PCSK9 through SREBP2 pathway? Int J Cardiol 207:361–362
Li H, Dong B, Park SW et al (2009) Hepatocyte nuclear factor 1α plays a critical role in PCSK9 gene transcription and regulation by the natural hypocholesterolemic compound berberine. J Biol Chem 284(42):28885–28895
Dullaart RP, Annema W, Tio RA et al (2014) The HDL anti-inflammatory function is impaired in myocardial infarction and may predict new cardiac events independent of HDL cholesterol. Clin Chim Acta 433(7):34–38
De Fabiani E, Mitro N, Anzulovich AC et al (2001) The negative effects of bile acids and tumor necrosis factor-α on the transcription of cholesterol 7α-hydroxylase gene (cyp7a1) converge to hepatic nuclear factor-4: a novel mechanism of feedback regulation of bile acid synthesis mediated by nuclear receptors. J Biol Chem 276(33):30708–30716
Field FJ, Watt K, Mathur SN (2010) TNF-α decreases ABCA1 expression and attenuates HDL cholesterol efflux in the human intestinal cell line Caco-2. J Lipid Res 51(6):1407–1415
Khovidhunkit W, Moser AH, Shigenaga JK et al (2001) Regulation of scavenger receptor class B type I in hamster liver and Hep3B cells by endotoxin and cytokines. J Lipid Res 42(10):1636–1644
Wiriyaphan C, Xiao H, Decker EA et al (2015) Chemical and cellular antioxidative properties of threadfin bream (Nemipterus spp.) surimi byproduct hydrolysates fractionated by ultrafiltration. Food Chem 167(11):7–15
Chi CF, Wang B, Wang YM et al (2015) Isolation and characterization of three antioxidant peptides from protein hydrolysate of bluefin leatherjacket (Navodon septentrionalis) heads. J Funct Foods 12:1–10
Feng LJ, Yu CH, Ying KJ et al (2011) Hypolipidemic and antioxidant effects of total flavonoids of Perilla Frutescens leaves in hyperlipidemia rats induced by high-fat diet. Food Res Int 44(1):404–409
Acknowledgements
This work was supported by National Natural Science Foundation of China (Nos. 31571825, 31271979, and 31201245) and Natural Science Foundation of Tianjin, China (No. 15JCYBJC30100).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xue, Z., Wang, R., Wen, H. et al. Biochanin A and CPe-III Peptide Improved Hepatic Inflammation by Regulating the Hepatic Lipid Metabolic Pathways in Diet-Induced Obese Mice. Trans. Tianjin Univ. 24, 234–243 (2018). https://doi.org/10.1007/s12209-018-0117-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12209-018-0117-y