Abstract
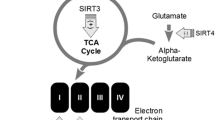
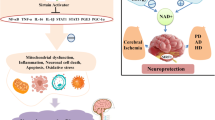
Sirtuins are a family of nicotinamide adenine dinucleotide (NAD+) dependent deacetylases involved in multiple biological functions including metabolism, inflammation, stress resistance and aging. In mammals, there are seven members (Sirt1—Sirt7), with diversities in their subcellular localizations and enzymatic activities. Here, we review the functions of sirtuins, with a focus on their roles in normal brain physiology such as neural development regulation, body homeostasis maintenance, and memory formation. We also discuss the role of sirtuins in a variety of brain diseases including stroke, Alzheimer’s, Parkinson’s, and motor neuron dysfunction. Because of the emerging functions of sirtuins in brain physiology and pathology, drugs targeting sirtuins may offer potential therapeutic values for brain disorders.
Similar content being viewed by others
References
Haigis M C, Sinclair D A. Mammalian sirtuins: Biological insights and disease relevance [J]. Annual Review of Pathology: Mechanisms of Disease, 2010, 5(1): 253–295.
Rine J, Herskowitz I. Four genes responsible for a position effect on expression from HML and HMR in saccharomyces cerevisiae [J]. Genetics, 1987, 116(1): 9–22.
Michishita E, Park J Y, Burneskis J M, et al. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins [J]. Molecular Biology of the Cell, 2005, 16(10): 4623–4635.
Vaziri H, Dessain S K, ng Eaton E, et al. HSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase [J]. Cell, 2001, 107(2): 149–159.
Hisahara S, Chiba S, Matsumoto H, et al. Histone deacetylase SIRT1 modulates neuronal differentiation by its nuclear translocation [J]. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(40): 15599–15604.
Sugino T, Maruyama M, Tanno M, et al. Protein deacetylase SIRT1 in the cytoplasm promotes nerve growth factor-induced neurite outgrowth in PC12 cells [J]. FEBS Letters, 2010, 584(13): 2821–2826.
North B J, Marshall B L, Borra M T, et al. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase [J]. Molecular Cell, 2003, 11(2): 437–444.
North B J, Verdin E. Interphase nucleocytoplasmic shuttling and localization of SIRT2 during mitosis [J]. PLoS One, 2007, 2(8): e784.
Verdin E, Hirschey M D, Finley LWS, et al. Sirtuin regulation of mitochondria: Energy production, apoptosis, and signaling [J]. Trends in Biochemical Sciences, 2010, 35(12): 669–675.
Michishita E, Mccord R A, Berber E, et al. SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin [J]. Nature, 2008, 452(7186): 492–496.
Ford E, Voit R, Liszt G, et al. Mammalian Sir2 homolog SIRT7 is an activator of RNA polymerase I transcription [J]. Genes & Development, 2006, 20(9): 1075–1080.
Onyango P, Celic I, Mccaffery J M, et al. SIRT3, a human SIR2 homologue, is an NAD-dependent deacetylase localized to mitochondria [J]. Proceedings of the National Academy of Sciences of the United States of America, 2002, 99(21): 13653–13658.
Barber M F, Michishita-Kioi E, Xi Y, et al. SIRT7 links H3K18 deacetylation to maintenance of oncogenic transformation [J]. Nature, 2012, 487(7405): 114–118.
Haigis M C, Mostoslavsky R, Haigis K M, et al. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells [J]. Cell, 2006, 126(5): 941–954.
Nakagawa T, Lomb D J, Haigis M C, et al. SIRT5 deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle [J]. Cell, 2009, 137(3): 560–570.
Du J, Zhou Y, Su X, et al. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase [J]. Science, 2011, 334(6057): 806–809.
Liszt G, Ford E, Kurtev M, et al. Mouse Sir2 homolog SIRT6 is a nuclear ADP-ribosyltransferase [J]. Journal of Biological Chemistry, 2005, 280(22): 21313–21320.
Jiang H, Khan S, Wang Y, et al. SIRT6 regulates TNF-α secretion through hydrolysis of longchain fatty acyl lysine [J]. Nature, 2013, 496(7443): 110–113.
Feldman J L, Baeza J, Denu J M. Activation of the protein deacetylase SIRT6 by long-chain fatty acids and widespread deacylation by mammalian sirtuins [J]. The Journal of Biological Chemistry, 2013, 288(43): 31350–31356.
Guarente L. Calorie restriction and sirtuins revisited [J]. Genes & Development, 2013, 27(19): 2072–2085.
Kaeberlein M, Mcvey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in saccharomyces cerevisiae by two different mechanisms [J]. Genes & Development, 1999, 13(19): 2570–2580.
Chen D, Guarente L. SIR2: A potential target for calorie restriction mimetics [J]. Trends in Molecular Medicine, 2007, 13(2): 64–71.
Kanfi Y, Naiman S, Amir G, et al. The sirtuin SIRT6 regulates lifespan in male mice [J]. Nature, 2012, 483(7388): 218–221.
Satoh A, Brace C S, Rensing N, et al. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH [J]. Cell Metabolism, 2013, 18(3): 416–430.
Hall J A, Dominy J E, Lee Y, et al. The sirtuin family’s role in aging and age-associated pathologies [J]. Journal of Clinical Investigation, 2013, 123(3): 973–979.
Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells [J]. Annual Review Neuroscience, 2009, 32(1): 149–184.
Prozorovski T, Schulze-Topphoff U, Glumm R, et al. Sirt1 contributes critically to the redoxdependent fate of neural progenitors [J]. Nature Cell Biology, 2008, 10(4): 385–394.
Tiberi L, van den Ameele J, Dimidschstein J, et al. BCL6 controls neurogenesis through Sirt1-dependent epigenetic repression of selective Notch targets [J]. Nature Neuroscience, 2012, 15(12): 1627–1635.
Ross S E, Greenberg M E, Stiles C D. Basic helix-loop-helix factors in cortical development [J]. Neuron, 2003, 39(1): 13–25.
Kageyama R, Ohtsuka T, Kobayashi T. The Hes gene family: Repressors and oscillators that orchestrate embryogenesis [J]. Development, 2007, 134(7): 1243–1251.
Ichi S, Boshnjaku V, Shen Y W, et al. Role of Pax3 acetylation in the regulation of Hes1 and Neurog2 [J]. Molecular Biology of the Cell, 2011, 22(4): 503–512.
Holloway K R, Calhoun T N, Saxena M, et al. SIRT1 regulates dishevelled proteins and promotes transient and constitutive Wnt signaling [J]. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(20): 9216–9221.
Liu B, Ghosh S, Yang X, et al. Resveratrol rescues SIRT1-dependent adult stem cell decline and alleviates progeroid features in laminopathy-based progeria [J]. Cell Metabolism, 2012, 16(6): 738–750.
Zhang Y, Wang J, Chen G, et al. Inhibition of Sirt1 promotes neural progenitors toward motoneuron differentiation from human embryonic stem cells [J]. Biochemical and Biophysical Research Communications, 2011, 404(2): 610–614.
Rafalski V A, Ho P P, Brett J O, et al. Expansion of oligodendrocyte progenitor cells following SIRT1 inactivation in the adult brain [J]. Nature Cell Biology, 2013, 15(6): 614–624.
Maxwell M M, Tomkinson E M, Nobles J, et al. The sirtuin 2 microtubule deacetylase is an abundant neuronal protein that accumulates in the aging CNS [J]. Human Molecular Genetics, 2011, 20(20): 3986–3996.
Werner H B, Kuhlmann K, Shen S, et al. Proteolipid protein is required for transport of sirtuin 2 into CNS myelin [J]. Journal of Neuroscience, 2007, 27(20): 7717–7730.
Li W, Zhang B, Tang J, et al. Sirtuin 2, a mammalian homolog of yeast silent information regulator-2 longevity regulator, is an oligodendroglial protein that decelerates cell differentiation through deacetylating α-tubulin [J]. The Journal of Neuroscience, 2007, 27(10): 2606–2616.
Ji S, Doucette J R, Nazarali A J. Sirt2 is a novel in vivo downstream target of Nkx2. 2 and enhances oligodendroglial cell differentiation [J]. Journal of Molecular Cell Biology, 2011, 3(6): 351–359.
Si X, Chen W, Guo X, et al. Activation of GSK3beta by Sirt2 is required for early lineage commitment of mouse embryonic stem cell [J]. PLoS One, 2013, 8(10): e76699.
Beirowski B, Gustin J, Armour S M, et al. Sirtwo-homolog 2 (Sirt2) modulates peripheral myelination through polarity protein Par-3/atypical protein kinase C (aPKC) signaling [J]. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(43): E952–961.
Komlos D, Mann K D, Zhuo Y, et al. Glutamate dehydrogenase 1 and SIRT4 regulate glial development [J]. Glia, 2013, 61(3): 394–408.
Guo W, Qian L, Zhang J, et al. Sirt1 overexpression in neurons promotes neurite outgrowth and cell survival through inhibition of the mTOR signaling [J]. Journal of Neuroscience Research, 2011, 89(11): 1723–1736.
Li X H, Chen C, Tu Y, et al. Sirt1 promotes axonogenesis by deacetylation of Akt and inactivation of GSK3 [J]. Molecular Neurobiology, 2013, 48(3): 490–499.
Liu C M, Wang R Y, Saijilafu , et al. MicroRNA-138 and SIRT1 form a mutual negative feedback loop to regulate mammalian axon regeneration [J]. Genes & Development, 2013, 27(13): 1473–1483.
Michan S, Li Y, Chou M M H, et al. SIRT1 is essential for normal cognitive function and synaptic plasticity [J]. The Journal of Neuroscience, 2010, 30(29): 9695–9707.
Codocedo J F, Allard C, Godoy J A, et al. SIRT1 regulates dendritic development in hippocampal neurons [J]. PLoS One, 2012, 7(10): e47073.
Coppari R. Metabolic actions of hypothalamic SIRT1 [J]. Trends in Endocrinology and Metabolism, 2012, 23(4): 179–185.
Ramadori G, Lee C E, Bookout A L, et al. Brain SIRT1: Anatomical distribution and regulation by energy availability [J]. The Journal of Neuroscience, 2008, 28(40): 9989–9996.
Ramadori G, Fujikawa T, Fukuda M, et al. SIRT1 deacetylase in POMC neurons is required for homeostatic defenses against diet-induced obesity [J]. Cell Metabolism, 2010, 12(1): 78–87.
Ramadori G, Fujikawa T, Erson J, et al. SIRT1 deacetylase in SF1 neurons protects against metabolic imbalance [J]. Cell Metabolism, 2011, 14(3): 301–312.
Hong S H, Lee K S, Kwak S J, et al. Minibrain/dyrk1a regulates food intake through the Sir2-FOXO-sNPF/NPY pathway in drosophila and mammals [J]. PLoS Genetics, 2012, 8(8): e1002857.
Cakir I, Perello M, Lansari O, et al. Hypothalamic Sirt1 regulates food intake in a rodent model system [J]. PLoS One, 2009, 4(12): e8322.
Veláquez D A, Martinez G, Romero A, et al. The central Sirtuin 1/p53 pathway is essential for the orexigenic action of ghrelin [J]. Diabetes, 2011, 60(4): 1177–1185.
Dietrich M O, Antunes C, Geliang G, et al. Agrp neurons mediate Sirt1’s action on the melanocortin system and energy balance: Roles for Sirt1 in neuronal firing and synaptic plasticity [J]. The Journal of Neuroscience, 2010, 30(35): 11815–11825.
Asher G, Gatfield D, Stratmann M, et al. SIRT1 regulates circadian clock gene expression through PER2 deacetylation [J]. Cell, 2008, 134(2): 317–328.
Nakahata Y, Kaluzova M, Grimaldi B, et al. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control [J]. Cell, 2008, 134(2): 329–340.
Chang H C, Guarente L. SIRT1 mediates central circadian control in the SCN by a mechanism that decays with aging [J]. Cell, 2013, 153(7): 1448–1460.
Panossian L, Fenik P, Zhu Y, et al. SIRT1 regulation of wakefulness and senescence-like phenotype in wake neurons [J]. The Journal of Neuroscience, 2011, 31(11): 4025–4036.
Peek C B, Affinati A H, Ramsey KM, et al. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice [J]. Science, 2013, 342(6158):1243417.
Monteserin-Garcia J, Al-Massadi O, Seoane L M, et al. Sirt1 inhibits the transcription factor CREB to regulate pituitary growth hormone synthesis [J]. FASEB Journal, 2013, 27(4): 1561–1571.
Schwer B, Schumacher B, Lombard D B, et al. Neural sirtuin 6 (Sirt6) ablation attenuates somatic growth and causes obesity [J]. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(50): 21790–21794.
Ferguson D, Koo J W, Feng J, et al. Essential role of SIRT1 signaling in the nucleus accumbens in cocaine and morphine action [J]. The Journal of Neuroscience, 2013, 33(41): 16088–16098.
Libert S, Pointer K, Bell E L, et al. SIRT1 activates MAO-A in the brain to mediate anxiety and exploratory drive [J]. Cell, 2011, 147(7): 1459–1472.
Gao J, Wang W Y, Mao Y W, et al. A novel pathway regulates memory and plasticity via SIRT1 and miR-134 [J]. Nature, 2010, 466(7310): 1105–1109.
Zhao Y N, Li W F, Li F, et al. Resveratrol improves learning and memory in normally aged mice through microRNA-CREB pathway [J]. Biochemical and Biophysical Research Communications, 2013, 435(4): 597–602.
Qiu X, Brown K, Hirschey M D, et al. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation [J]. Cell Metabolism, 2010, 12(6): 662–667.
Lin Z F, Xu H B, Wang J Y, et al. SIRT5 desuccinylates and activates SOD1 to eliminate ROS [J]. Biochemical and Biophysical Research Communications, 2013, 441(1): 191–195.
Hsu C P, Zhai P, Yamamoto T, et al. Silent information regulator 1 protects the heart from ischemia/reperfusion [J]. Circulation, 2010, 122(21): 2170–2182.
Nadtochiy S M, Yao H, Mcburney M W, et al. SIRT1-mediated acute cardioprotection [J]. American Journal of Physiology-Heart and Circulatory Physiology, 2011, 301(4): H1506–H1512.
Sundaresan N R, Gupta M, Kim G, et al. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice [J]. The Journal of Clinical Investigation, 2009, 119(9): 2758–2771.
Sundaresan N R, Vasudevan P, Zhong L, et al. The sirtuin SIRT6 blocks IGF-Akt signaling and development of cardiac hypertrophy by targeting c-Jun [J]. Nature Medicine, 2012, 18(11): 1643–1650.
Vakhrusheva O, Smolka C, Gajawada P, et al. Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice [J]. Circulation Research, 2008, 102(6): 703–710.
Narayan N, Lee I H, Borenstein R, et al. The NAD-dependent deacetylase SIRT2 is required for programmed necrosis [J]. Nature, 2012, 492(7428): 199–204.
Newton K, Hildebrand J M, Shen Z, et al. Is SIRT2 required for necroptosis? [J]. Nature, 2014, 506(7489): E4–E6.
Narayan N, Lee I H, Borenstein R, et al. Retraction: The NAD-dependent deacetylase SIRT2 is required for programmed necrosis [J]. Nature, 2014, 506(7489): 516.
Morris K C, Lin H W, Thompson J W, et al. Pathways for ischemic cytoprotection: Role of sirtuins in caloric restriction, resveratrol, and ischemic preconditioning [J]. Journal of Cerebral Blood Flow & Metabolism, 2011, 31(4): 1003–1019.
Della-Morte D, Dave K R, Defazio R A, et al. Resveratrol pretreatment protects rat brain from cerebral ischemic damage via a sirtuin 1-uncoupling protein 2 pathway [J]. Neuroscience, 2009, 159(3): 993–1002.
Clark D, Tuor U I, Thompson R, et al. Protection against recurrent stroke with resveratrol:Endothelial protection [J]. PLoS One, 2012, 7(10): e47792.
Wang L, Zhang L, Chen Z B, et al. Icariin enhances neuronal survival after oxygen and glucose deprivation by increasing SIRT1 [J]. European Journal of Pharmacology, 2009, 609(1–3): 40–44.
Zhu H R, Wang Z Y, Zhu X L, et al. Icariin protects against brain injury by enhancing SIRT1-dependent PGC-1α expression in experimental stroke [J]. Neuropharmacology, 2010, 59(1–2): 70–76.
Raval A P, Dave K R, Péez-Pinzón M A. Resveratrol mimics ischemic preconditioning in the brain [J]. Journal of Cerebral Blood Flow & Metabolism, 2006, 26(9): 1141–1147.
Yan W, Fang Z, Yang Q, et al. SirT1 mediates hyperbaric oxygen preconditioning-induced ischemic tolerance in rat brain [J]. Journal of Cerebral Blood Flow & Metabolism, 2013, 33(3): 396–406.
Wang P, Xu T Y, Guan Y F, et al. Nicotinamide phosphoribosyltransferase protects against ischemic stroke through SIRT1-dependent adenosine monophosphate-activated kinase pathway [J]. Annals of Neurology, 2011, 69(2): 360–374.
Wang P, Guan Y F, Du H, et al. Induction of autophagy contributes to the neuroprotection of nicotinamide phosphoribosyltransferase in cerebral ischemia [J]. Autophagy, 2012, 8(1): 77–87.
Hernández-Jiménez M, Hurtado O, Cuartero M I, et al. Silent information regulator 1 protects the brain against cerebral ischemic damage [J]. Stroke, 2013, 44(8): 2333–2337.
Lee O H, Kim J, Kim J M, et al. Decreased expression of sirtuin 6 is associated with release of high mobility group box-1 after cerebral ischemia [J]. Biochemical and Biophysical Research Communications, 2013, 438(2): 388–394.
Qin W, Yang T, Ho L, et al. Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction [J]. The Journal of Biological Chemistry, 2006, 281(31): 21745–21754.
Kim D, Nguyen M D, Dobbin M M, et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis [J]. The EMBO Journal, 2007, 26(13): 3169–3179.
Green K N, Steffan J S, Martinez-Coria H, et al. Nicotinamide restores cognition in Alzheimer’s disease transgenic mice via a mechanism involving sirtuin inhibition and selective reduction of Thr231-phosphotau [J]. The Journal of Neuroscience, 2008, 28(45): 11500–11510.
Donmez G, Wang D, Cohen D E, et al. SIRT1 suppresses beta-amyloid production by activating the α-secretase gene ADAM10 [J]. Cell, 2010, 142(2): 320–332.
Wang R, Li J J, Diao S, et al. Metabolic stress modulates Alzheimer’s β-secretase gene transcription via SIRT1-PPARΓ-PGC-1 in neurons [J]. Cell Metabolism, 2013, 17(5): 685–694.
Chen J, Zhou Y, Mueller-Steiner S, et al. SIRT1 protects against microglia-dependent amyloid-β toxicity through inhibiting NF-κB signaling [J]. The Journal of Biological Chemistry, 2005, 280(48): 40364–40374.
Min S W, Cho S H, Zhou Y, et al. Acetylation of tau inhibits its degradation and contributes to tauopathy [J]. Neuron, 2010, 67(6): 953–966.
Kumar R, Chaterjee P, Sharma P K, et al. Sirtuin1: A promising serum protein marker for early detection of Alzheimer’s disease [J]. PLoS One, 2013, 8(4): e61560.
Porcelli S, Salfi R, Politis A, et al. Association between sirtuin2 gene rs10410544 polymorphism and depression in Alzheimer’s disease in two independent European samples [J]. Journal of Neural Transmission, 2013, 120(12): 1709–1715.
Wei W, Xu X, Li H, et al. The SIRT2 polymorphism rs10410544 and risk of Alzheimer’s disease: A meta-analysis [J]. Neuromolecular Medicine, 2014. DOI 10.1007/s12017-014-8291-0 (published online).
Xia M, Yu J T, Miao D, et al. SIRT2 polymorphism rs10410544 is associated with Alzheimer’s disease in a Han Chinese population [J]. Journal of Neurological Sciences, 2014, 336(1–2): 48–51.
Polito L, Kehoe P G, Davin A, et al. The SIRT2 polymorphism rs10410544 and risk of Alzheimer’s disease in two Caucasian case-control cohorts [J]. Alzheimers & Dementia, 2013, 9(4): 392–399.
Rothgiesser K M, Erener S, Waibel S, et al. SIRT2 regulates NF-κB-dependent gene expression through deacetylation of p65 Lys310 [J]. Journal of Cell Science, 2010, 123(24): 4251–4258.
Pais T F, Szego E M, Marques O, et al. The NAD-dependent deacetylase sirtuin 2 is a suppressor of microglial activation and brain inflammation [J]. The EMBO Journal, 2013, 32(19): 2603–2616.
Weir H J M, Murray T K, Kehoe P G, et al. CNS SIRT3 expression is altered by reactive oxygen species and in Alzheimer’s disease [J]. PLoS One, 2012, 7(11): e48225.
Albani D, Polito L, Batelli S, et al. The SIRT1 activator resveratrol protects SK-N-BE cells from oxidative stress and against toxicity caused by α-synuclein or amyloid-β (1–42) peptide [J]. Journal of Neurochemistry, 2009, 110(5): 1445–1456.
Wu Y, Li X, Zhu J X, et al. Resveratrol-activated AMPK/SIRT1/autophagy in cellular models of Parkinson’s disease [J]. Neurosignals, 2011, 19(3): 163–174.
Blanchet J, Longpre F, Bureau G, et al. Resveratrol, a red wine polyphenol, protects dopaminergic neurons in MPTP-treated mice [J]. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 2008, 32(5): 1243–1250.
Donmez G, Arun A, Chung C Y, et al. SIRT1 protects against α-synuclein aggregation by activating molecular chaperones [J]. The Journal of Neuroscience, 2012, 32(1): 124–132.
Outeiro T F, Kontopoulos E, Altmann S M, et al. Sirtuin 2 inhibitors rescue α-synuclein-mediated toxicity in models of Parkinson’s disease [J]. Science, 2007, 317(5837): 516–519.
Sampaio-Marques B, Felgueiras C, Silva A, et al. SNCA (α-synuclein)-induced toxicity in yeast cells is dependent on sirtuin 2 (Sir2)-mediated mitophagy [J]. Autophagy, 2012, 8(10): 1494–1509.
Liu L, Arun A, Ellis L, et al. Sirtuin 2 (SIRT2) enhances 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP)-induced nigrostriatal damage via deacetylating forkhead box O3a (Foxo3a) and activating Bim protein [J]. The Journal of Biological Chemistry, 2012, 287(39): 32307–32311.
Liu L, Arun A, Ellis L, et al. Additons and corrections: Sirtuin 2 (SIRT2) enhances 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP)-induced nigrostriatal damage via deacetylating forkhead box O3a (Foxo3a) and activating Bim protein [J]. The Journal of Biological Chemistry, 2013, 288(33): 24163.
Glorioso C, Oh S, Douillard G G, et al. Brain molecular aging, promotion of neurological disease and modulation by Sirtuin5 longevity gene polymorphism [J]. Neurobiology of Disease, 2011, 41(2): 279–290.
Pallos J, Bodai L, Lukacsovich T, et al. Inhibition of specific HDACs and sirtuins suppresses pathogenesis in a drosophila model of Huntington’s disease [J]. Human Molecular Genetics, 2008, 17(33): 3767–3775.
Parker J A, Arango M, Abderrahmane S, et al. Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons [J]. Nature Genetics, 2005, 37(4): 349–350.
Jiang M, Wang J, Fu J, et al. Neuroprotective role of Sirt1 in mammalian models of Huntington’s disease through activation of multiple Sirt1 targets [J]. Nature Medicine, 2012, 18(1): 153–158.
Jeong H, Cohen D E, Cui L, et al. Sirt1 mediates neuroprotection from mutant huntingtin by activation of the TORC1 and CREB transcriptional pathway [J]. Nature Medicine, 2012, 18(1): 159–165.
Luthi-Carter R, Taylor D M, Pallos J, et al. SIRT2 inhibition achieves neuroprotection by decreasing sterol biosynthesis [J]. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(17): 7927–7932.
Chopra V, Quinti L, Kim J, et al. The sirtuin 2 inhibitor AK-7 is neuroprotective in Huntington’s disease mouse models [J]. Cell Reports, 2012, 2(6): 1492–1497.
Bobrowska A, Donmez G, Weiss A, et al. SIRT2 ablation has no effect on tubulin acetylation in brain, cholesterol biosynthesis or the progression of Huntington’s disease phenotypes in vivo [J]. PLoS One, 2012, 7(4): e34805.
Fu J, Jin J, Cichewicz R H, et al. Trans-(-)-epsilonviniferin increases mitochondrial sirtuin3 (SIRT3), activates AMP-activated protein kinase (AMPK), and protects cells in models of Huntington disease [J]. The Journal of Biological Chemistry, 2012, 287(29): 24460–24472.
Dobbin M M, Madabhushi R, Pan L, et al. SIRT1 collaborates with ATM and HDAC1 to maintain genomic stability in neurons [J]. Nature Neuroscience, 2013, 16(8): 1008–1015.
Li Y, Xu W, Mcburney M W, et al. SirT1 inhibition reduces IGF-I/IRS-2/Ras/ERK1/2 signaling and protects neurons [J]. Cell Metabolism, 2008, 8(1): 38–48.
Kim S H, Lu H F, Alano C C. Neuronal Sirt3 protects against excitotoxic injury in mouse cortical neuron culture [J]. PLoS One, 2011, 6(3): e14731.
Someya S, Yu W, Hallows W C, et al. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction [J]. Cell, 2010, 143(5): 802–812.
Wang J, Zhang Y, Tang L, et al. Protective effects of resveratrol through the up-regulation of SIRT1 expression in the mutant hSOD1-G93A-bearing motor neuron-like cell culture model of amyotrophic lateral sclerosis [J]. Neuroscience Letters, 2011, 503(3): 250–255.
Araki T, Sasaki Y, Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration [J]. Science, 2004, 305(5686): 1010–1013.
Suzuki K, Koike T. Mammalian Sir2-related protein (SIRT) 2-mediated modulation of resistance to axonal degeneration in slow Wallerian degeneration mice: A crucial role of tubulin deacetylation [J]. Neuroscience, 2007, 147(3): 599–612.
Nimmagadda V K, Bever C T, Vattikunta N R, et al. Overexpression of SIRT1 protein in neurons protects against experimental autoimmune encephalomyelitis through activation of multiple SIRT1 targets [J]. The Journal of Immunology, 2013, 190(9): 4595–4607.
Bizat N, Peyrin J M, Haik S, et al. Neuron dysfunction is induced by prion protein with an insertional mutation via a Fyn kinase and reversed by sirtuin activation in caenorhabditis elegans [J]. The Journal of Neuroscience, 2010, 30(15): 5394–5403.
Seo J S, Moon M H, Jeong J K, et al. SIRT1, a histone deacetylase, regulates prion protein-induced neuronal cell death [J]. Neurobiology of Aging, 2012, 33(6): 1110–1120.
Jeong J K, Moon M H, Lee Y J, et al. Autophagy induced by the class III histone deacetylase Sirt1 prevents prion peptide neurotoxicity [J]. Neurobiology of Aging, 2013, 34(1): 146–156.
Bodkin N L, Alexander T M, Ortmeyer H K, et al. Mortality and morbidity in laboratory-maintained Rhesus monkeys and effects of long-term dietary restriction [J]. Journals of Gerontology Series A: Biological Sciences & Medical Sciences, 2003, 58(3): 212–219.
Mattison J A, Roth G S, Beasley T M, et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study [J]. Nature, 2012, 489(7415): 318–321.
Kim H S, Xiao C, Wang R H, et al. Hepatic-specific disruption of SIRT6 in mice results in fatty liver formation due to enhanced glycolysis and triglyceride synthesis [J]. Cell Metabolism, 2010, 12(3): 224–236.
Shao J, Liu T, Xie Q R, et al. Adjudin attenuates lipopolysaccharide (LPS)- and ischemia-induced microglial activation [J]. Journal of Neuroimmunology, 2013, 254(1–2): 83–90.
Mouchiroud L, Houtkooper R H, Moullan N, et al. The NAD+/sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling [J]. Cell, 2013, 154(2): 430–441.
Gomes A P, Price N L, Ling A J Y, et al. Declining NAD+ induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging [J]. Cell, 2013, 155(7): 1624–1638.
Yoshino J, Mills K F, Yoon M J, et al. Nicotinamide mononucleotide, a key NAD+ intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice [J]. Cell Metabolism, 2011, 14(4): 528–536.
Canto C, Houtkooper R H, Pirinen E, et al. The NAD+ precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat dietinduced obesity [J]. Cell Metabolism, 2012, 15(6): 838–847.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: the National Natural Science Foundation of China (No. 31270032), the Shanghai Jiao Tong University Interdisciplinary Research Grant (No. YG2012ZD05) and the Grant from Ministry of Science and Technology of China (No. 2013CB945604)
Rights and permissions
About this article
Cite this article
Shao, Jx., Zhang, Tt., Liu, Ty. et al. Sirtuin functions in the brain: From physiological to pathological aspects. J. Shanghai Jiaotong Univ. (Sci.) 19, 651–662 (2014). https://doi.org/10.1007/s12204-014-1562-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12204-014-1562-y