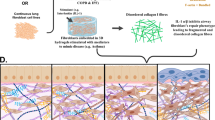
Abstract
Idiopathic pulmonary fibrosis is a chronic disease characterized by progressive lung scarring that inhibits gas exchange. Evidence suggests fibroblast-matrix interactions are a prominent driver of disease. However, available preclinical models limit our ability to study these interactions. We present a technique for synthesizing phototunable poly(ethylene glycol) (PEG)-based hybrid-hydrogels comprising healthy or fibrotic decellularized extracellular matrix (dECM) to decouple mechanical properties from composition and elucidate their roles in fibroblast activation. Here, we engineered and characterized phototunable hybrid-hydrogels using molecular techniques such as ninhydrin and Ellman’s assays to assess dECM functionalization, and parallel-plate rheology to measure hydrogel mechanical properties. These biomaterials were employed to investigate the activation of fibroblasts from dual-transgenic Col1a1-GFP and αSMA-RFP reporter mice in response to changes in composition and mechanical properties. We show that reacting functionalized dECM from healthy or bleomycin-injured mouse lungs with PEG alpha-methacrylate (αMA) in an off-stoichiometry Michael-addition reaction created soft hydrogels mimicking a healthy lung elastic modulus (4.99 ± 0.98 kPa). Photoinitiated stiffening increased the material modulus to fibrotic values (11.48 ± 1.80 kPa). Percent activation of primary murine fibroblasts expressing Col1a1 and αSMA increased by approximately 40% following dynamic stiffening of both healthy and bleomycin hybrid-hydrogels. There were no significant differences between fibroblast activation on stiffened healthy versus stiffened bleomycin-injured hybrid-hydrogels. Phototunable hybrid-hydrogels provide an important platform for probing cell-matrix interactions and developing a deeper understanding of fibrotic activation in pulmonary fibrosis. Our results suggest that mechanical properties are a more significant contributor to fibroblast activation than biochemical composition within the scope of the hybrid-hydrogel platform evaluated in this study.





Similar content being viewed by others
Data Availability
The raw and processed data required to reproduce these findings are available here: Saleh, Kamiel; Hewawasam, Rukshika; Šerbedžija, Predrag; Blomberg, Rachel; Noreldeen, Saif; Edelman, Benjamin; Smith, Bradford; Riches, David; Magin, Chelsea (2022), “Engineering hybrid-hydrogels comprised of healthy or diseased decellularized extracellular matrix to study pulmonary fibrosis”, Mendeley Data, V1, https://doi.org/10.17632/stxpj6xmgg.1.
References
Arkenberg, M. R., H. D. Nguyen, and C. C. Lin. Recent advances in bio-orthogonal and dynamic crosslinking of biomimetic hydrogels. J. Mater. Chem. B. 8:7835–7855, 2020.
Balestrini, J. L., S. Chaudhry, V. Sarrazy, A. Koehler, and B. Hinz. The mechanical memory of lung myofibroblasts. Integr. Biol. (Camb). 4:410–421, 2012.
Barbarin, V., A. Nihoul, P. Misson, M. Arras, M. Delos, I. Leclercq, D. Lison, and F. Huaux. The role of pro- and anti-inflammatory responses in silica-induced lung fibrosis. Respir. Res. 6:112, 2005.
Bolukbas, D. A., M. M. De Santis, H. N. Alsafadi, A. Doryab, and D. E. Wagner. The preparation of decellularized mouse lung matrix scaffolds for analysis of lung regenerative cell potential. Methods Mol. Biol. 275–295:2019, 1940.
Booth, A. J., R. Hadley, A. M. Cornett, A. A. Dreffs, S. A. Matthes, J. L. Tsui, K. Weiss, J. C. Horowitz, V. F. Fiore, T. H. Barker, B. B. Moore, F. J. Martinez, L. E. Niklason, and E. S. White. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am. J. Respir. Crit. Care Med. 186:866–876, 2012.
Christensen, P. J., R. E. Goodman, L. Pastoriza, B. Moore, and G. B. Toews. Induction of lung fibrosis in the mouse by intratracheal instillation of fluorescein isothiocyanate is not T-cell-dependent. Am. J. Pathol. 155:1773–1779, 1999.
Crapo, P. M., T. W. Gilbert, and S. F. Badylak. An overview of tissue and whole organ decellularization processes. Biomaterials. 32:3233–3243, 2011.
de Hilster, R. H. J., P. K. Sharma, M. R. Jonker, E. S. White, E. A. Gercama, M. Roobeek, W. Timens, M. C. Harmsen, M. N. Hylkema, and J. K. Burgess. Human lung extracellular matrix hydrogels resemble the stiffness and viscoelasticity of native lung tissue. Am. J. Physiol. Lung Cell. Mol. Physiol. 318:L698–L704, 2020.
DellaLatta, V., A. Cecchettini, S. DelRy, and M. A. Morales. Bleomycin in the setting of lung fibrosis induction: from biological mechanisms to counteractions. Pharmacol. Res. 97:122–130, 2015.
Dolhnikoff, M., T. Mauad, and M. S. Ludwig. Extracellular matrix and oscillatory mechanics of rat lung parenchyma in bleomycin-induced fibrosis. Am. J. Respir. Crit. Care Med. 160:1750–1757, 1999.
Gillette, B. M., J. A. Jensen, M. Wang, J. Tchao, and S. K. Sia. Dynamic hydrogels: switching of 3D microenvironments using two-component naturally derived extracellular matrices. Adv. Mater. 22:686–691, 2010.
Haak, A. J., Q. Tan, and D. J. Tschumperlin. Matrix biomechanics and dynamics in pulmonary fibrosis. Matrix Biol. 73:64–76, 2018.
Heinzelmann, K., M. Lehmann, M. Gerckens, N. Noskovicova, M. Frankenberger, M. Lindner, R. Hatz, J. Behr, A. Hilgendorff, M. Konigshoff, and O. Eickelberg. Cell-surface phenotyping identifies CD36 and CD97 as novel markers of fibroblast quiescence in lung fibrosis. Am. J. Physiol. Lung Cell. Mol.r Physiol. 315:L682–L696, 2018.
Herrera, J., C. A. Henke, and P. B. Bitterman. Extracellular matrix as a driver of progressive fibrosis. J. Clin. Invest. 128:45–53, 2018.
Jones, M. G., A. Fabre, P. Schneider, F. Cinetto, G. Sgalla, M. Mavrogordato, S. Jogai, A. Alzetani, B. G. Marshall, K. M. O’Reilly, J. A. Warner, P. M. Lackie, D. E. Davies, D. M. Hansell, A. G. Nicholson, I. Sinclair, K. K. Brown, and L. Richeldi. Three-dimensional characterization of fibroblast foci in idiopathic pulmonary fibrosis. JCI Insight. 2016. https://doi.org/10.1172/jci.insight.86375.
Keith, R. C., J. L. Powers, E. F. Redente, A. Sergew, R. J. Martin, A. Gizinski, V. M. Holers, S. Sakaguchi, and D. W. Riches. A novel model of rheumatoid arthritis-associated interstitial lung disease in SKG mice. Exp. Lung Res. 38:55–66, 2012.
Knudsen, L., E. Lopez-Rodriguez, L. Berndt, L. Steffen, C. Ruppert, J. H. T. Bates, M. Ochs, and B. J. Smith. Alveolar micromechanics in bleomycin-induced lung injury. Am. J. Respir. Cell Mol. Biol. 59:757–769, 2018.
Korfei, M., S. Schmitt, C. Ruppert, I. Henneke, P. Markart, B. Loeh, P. Mahavadi, M. Wygrecka, W. Klepetko, L. Fink, P. Bonniaud, K. T. Preissner, G. Lochnit, L. Schaefer, W. Seeger, and A. Guenther. Comparative proteomic analysis of lung tissue from patients with idiopathic pulmonary fibrosis (IPF) and lung transplant donor lungs. J. Proteome Res. 10:2185–2205, 2011.
Liu, F., C. M. Haeger, P. B. Dieffenbach, D. Sicard, I. Chrobak, A. M. F. Coronata, M. M. S. Velandia, S. Vitali, R. A. Colas, P. C. Norris, A. Marinkovic, X. L. Liu, J. Ma, C. D. Rose, S. J. Lee, S. A. A. Comhair, S. C. Erzurum, J. D. McDonald, C. N. Serhan, S. R. Walsh, D. J. Tschumperlin, and L. E. Fredenburgh. Distal vessel stiffening is an early and pivotal mechanobiological regulator of vascular remodeling and pulmonary hypertension. JCI Insight. 2016. https://doi.org/10.1172/jci.insight.86987.
Liu, F., D. Lagares, K. M. Choi, L. Stopfer, A. Marinkovic, V. Vrbanac, C. K. Probst, S. E. Hiemer, T. H. Sisson, J. C. Horowitz, I. O. Rosas, L. E. Fredenburgh, C. Feghali-Bostwick, X. Varelas, A. M. Tager, and D. J. Tschumperlin. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 308:L344–L357, 2015.
Liu, F., and D. J. Tschumperlin. Micro-mechanical characterization of lung tissue using atomic force microscopy. J. Vis. Exp. 2011. https://doi.org/10.3791/2911.
Liu, S. S., C. Liu, X. X. Lv, B. Cui, J. Yan, Y. X. Li, K. Li, F. Hua, X. W. Zhang, J. J. Yu, J. M. Yu, F. Wang, S. Shang, P. P. Li, Z. G. Zhou, Y. Xiao, and Z. W. Hu. The chemokine CCL1 triggers an AMFR-SPRY1 pathway that promotes differentiation of lung fibroblasts into myofibroblasts and drives pulmonary fibrosis. Immunity. 54:2433–2435, 2021.
Manali, E. D., C. Moschos, C. Triantafillidou, A. Kotanidou, I. Psallidas, S. P. Karabela, C. Roussos, S. Papiris, A. Armaganidis, G. T. Stathopoulos, and N. A. Maniatis. Static and dynamic mechanics of the murine lung after intratracheal bleomycin. BMC Pulm. Med. 11:33, 2011.
Meyvis, T. K. L., S. C. De Smedt, J. Demeester, and W. E. Hennink. Rheological monitoring of long-term degrading polymer hydrogels. J. Rheol. 43:933–950, 1999.
Moore, B. B., and C. M. Hogaboam. Murine models of pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 294:L152-160, 2008.
National Research Council (U.S.). Committee for the update of the guide for the care and use of laboratory animals, Institute for Laboratory Animal Research (U.S.) and National Academies Press (U.S.). Guide for the care and use of laboratory animals. Washington, D.C.: National Academies Press, 2011, p. xxv, 220 p.
Nizamoglu, M., R. H. J. de Hilster, F. Zhao, P. K. Sharma, T. Borghuis, O. M. C. Harmsen, and J. K. Burgess. An in vitro model of fibrosis using crosslinked native extracellular matrix-derived hydrogels to modulate biomechanics without changing composition. bioRxiv. 2022. https://doi.org/10.1101/2022.02.02.478812.
Parker, M. W., D. Rossi, M. Peterson, K. Smith, K. Sikstrom, E. S. White, J. E. Connett, C. A. Henke, O. Larsson, and P. B. Bitterman. Fibrotic extracellular matrix activates a profibrotic positive feedback loop. J. Clin. Investig. 124:1622–1635, 2014.
Petrou, C. L., T. J. D’Ovidio, D. A. Bolukbas, S. Tas, R. D. Brown, A. Allawzi, S. Lindstedt, E. Nozik-Grayck, K. R. Stenmark, D. E. Wagner, and C. M. Magin. Clickable decellularized extracellular matrix as a new tool for building hybrid-hydrogels to model chronic fibrotic diseases in vitro. J. Mater. Chem. B. 8:6814–6826, 2020.
Pouliot, R. A., P. A. Link, N. S. Mikhaiel, M. B. Schneck, M. S. Valentine, F. J. Kamga Gninzeko, J. A. Herbert, M. Sakagami, and R. L. Heise. Development and characterization of a naturally derived lung extracellular matrix hydrogel. J. Biomed. Mater. Res. A. 104:1922–1935, 2016.
Redente, E. F., B. P. Black, D. S. Backos, A. N. Bahadur, S. M. Humphries, D. A. Lynch, R. M. Tuder, R. L. Zemans, and D. W. H. Riches. Persistent, progressive pulmonary fibrosis and epithelial remodeling in mice. Am. J. Respir. Cell. Mol. Biol. 64:669–676, 2021.
Richeldi, L., H. R. Collard, and M. G. Jones. Idiopathic pulmonary fibrosis. Lancet. 389:1941–1952, 2017.
Rosales, A. M., S. L. Vega, F. W. DelRio, J. A. Burdick, and K. S. Anseth. Hydrogels with reversible mechanics to probe dynamic cell microenvironments. Angew. Chem. Int. Ed. Engl. 56:12132–12136, 2017.
Rube, C. E., D. Uthe, K. W. Schmid, K. D. Richter, J. Wessel, A. Schuck, N. Willich, and C. Rube. Dose-dependent induction of transforming growth factor beta (TGF-beta) in the lung tissue of fibrosis-prone mice after thoracic irradiation. Int. J. Radiat. Oncol. Biol. Phys. 47:1033–1042, 2000.
Saldin, L. T., M. C. Cramer, S. S. Velankar, L. J. White, and S. F. Badylak. Extracellular matrix hydrogels from decellularized tissues: structure and function. Acta Biomater. 49:1–15, 2017.
Sava, P., A. Ramanathan, A. Dobronyi, X. Peng, H. Sun, A. Ledesma-Mendoza, E. L. Herzog, and A. L. Gonzalez. Human pericytes adopt myofibroblast properties in the microenvironment of the IPF lung. JCI Insight. 2:e96352, 2017.
Schiller, H. B., I. E. Fernandez, G. Burgstaller, C. Schaab, R. A. Scheltema, T. Schwarzmayr, T. M. Strom, O. Eickelberg, and M. Mann. Time- and compartment-resolved proteome profiling of the extracellular niche in lung injury and repair. Mol. Syst. Biol. 11:819, 2015.
Shi, X., Y. Chen, Q. Liu, X. Mei, J. Liu, Y. Tang, R. Luo, D. Sun, Y. Ma, W. Wu, W. Tu, Y. Zhao, W. Xu, Y. Ke, S. Jiang, Y. Huang, R. Zhang, L. Wang, Y. Chen, J. Xia, W. Pu, H. Zhu, X. Zuo, Y. Li, J. Xu, F. Gao, D. Wei, J. Chen, W. Yin, Q. Wang, H. Dai, L. Yang, G. Guo, J. Cui, N. Song, H. Zou, S. Zhao, J. H. W. Distler, L. Jin, and J. Wang. LDLR dysfunction induces LDL accumulation and promotes pulmonary fibrosis. Clin. Transl. Med.12:e711, 2022.
Silver, F. H., and D. E. Birk. Molecular-structure of collagen in solution—comparison of type-I, type-Ii, type-Iii and type-V. Int. J. Biol. Macromol. 6:125–132, 1984.
Smith, B. J., G. S. Roy, A. Cleveland, C. Mattson, K. Okamura, C. M. Charlebois, K. L. Hamlington, M. V. Novotny, L. Knudsen, M. Ochs, R. D. Hite, and J. H. T. Bates. Three alveolar phenotypes govern lung function in murine ventilator-induced lung injury. Front. Physiol. 11:660, 2020.
Tsukui, T., K. H. Sun, J. B. Wetter, J. R. Wilson-Kanamori, L. A. Hazelwood, N. C. Henderson, T. S. Adams, J. C. Schupp, S. D. Poli, I. O. Rosas, N. Kaminski, M. A. Matthay, P. J. Wolters, and D. Sheppard. Collagen-producing lung cell atlas identifies multiple subsets with distinct localization and relevance to fibrosis. Nat. Commun. 11:1920, 2020.
Wagner, D. E., N. R. Bonenfant, D. Sokocevic, M. J. DeSarno, Z. D. Borg, C. S. Parsons, E. M. Brooks, J. J. Platz, Z. I. Khalpey, D. M. Hoganson, B. Deng, Y. W. Lam, R. A. Oldinski, T. Ashikaga, and D. J. Weiss. Three-dimensional scaffolds of acellular human and porcine lungs for high throughput studies of lung disease and regeneration. Biomaterials. 35:2664–2679, 2014.
Wang, Y., L. Zhang, T. Huang, G. R. Wu, Q. Zhou, F. X. Wang, L. M. Chen, F. Sun, Y. Lv, F. Xiong, S. Zhang, Q. Yu, P. Yang, W. Gu, Y. Xu, J. Zhao, H. Zhang, W. Xiong, and C. Y. Wang. The methyl-CpG-binding domain 2 facilitates pulmonary fibrosis by orchestrating fibroblast to myofibroblast differentiation. Eur. Respir. J. 2022. https://doi.org/10.1183/13993003.03697-2020.
Wolters, P. J., H. R. Collard, and K. D. Jones. Pathogenesis of idiopathic pulmonary fibrosis. Annu. Rev. Pathol. 9:157–179, 2014.
Wu, S. M., J. J. Tsai, H. C. Pan, J. L. Arbiser, L. Elia, and M. L. Sheu. Aggravation of pulmonary fibrosis after knocking down the aryl hydrocarbon receptor in the insulin-like growth factor 1 receptor pathway. Br. J. Pharmacol. 179:3430–3451, 2022.
Zhang, Y., M. Jiang, M. Nouraie, M. G. Roth, T. Tabib, S. Winters, X. Chen, J. Sembrat, Y. Chu, N. Cardenes, R. M. Tuder, E. L. Herzog, C. Ryu, M. Rojas, R. Lafyatis, K. F. Gibson, J. F. McDyer, D. J. Kass, and J. K. Alder. GDF15 is an epithelial-derived biomarker of idiopathic pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 317:L510–L521, 2019.
Acknowledgments
This work was supported by funding from the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) under Awards R01 HL080396 (CMM), R01 HL153096 (CMM, KSS, PS, and DWHR), R01HL151630 (BJS), and T32 HL 07085 (RB); the National Cancer Institute of the NIH under Award R21 CA252172 (CMM and RB); the National Science Foundation under Award 1941401 (CMM and RH); the Department of the Army under Award W81XWH-20-1-0037 (CMM).
Author information
Authors and Affiliations
Contributions
KSS, RH, PS, RB, BJS, DWHR, and CMM conceived the research plan. KSS, RH, PS, RB, BJS, SEN, and BE carried out experiments. All authors wrote, reviewed, and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Dr. Magin is an inventor on a pending patent related to the technology described in this manuscript. All remaining authors (KSS, RH, PS, RB, BJS, SEN, BE, and DWHR) have no conflicts of interest to disclose.
Human Studies
No human studies were carried out by the authors for this article.
Animal Studies
All institutional and national guidelines for the care and use of laboratory animals were followed and approved by the appropriate institutional committees.
Additional information
Associate Editor Cheng Dong oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Saleh, K.S., Hewawasam, R., Šerbedžija, P. et al. Engineering Hybrid-Hydrogels Comprised of Healthy or Diseased Decellularized Extracellular Matrix to Study Pulmonary Fibrosis. Cel. Mol. Bioeng. 15, 505–519 (2022). https://doi.org/10.1007/s12195-022-00726-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-022-00726-y