Abstract
Introduction
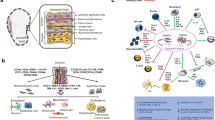
Chronic wounds remain a major clinical challenge. Human cryopreserved viable amniotic membrane (hCVAM) is among the most successful therapies, but the mechanisms of action remain loosely defined. Because proper regulation of macrophage behavior is critical for wound healing with biomaterial therapies, we hypothesized that hCVAM would positively regulate macrophage behavior in vitro, and that soluble factors released from the hCVAM would be important for this effect.
Materials and Methods
Primary human pro-inflammatory (M1) macrophages were seeded directly onto intact hCVAM or cultured in separation via transwell inserts (Soluble Factors) in the presence of pro-inflammatory stimuli (interferon-γ and lipopolysaccharide) to simulate the chronic wound environment. Macrophages were characterized after 1 and 6 days using multiplex gene expression analysis of 37 macrophage phenotype- and angiogenesis-related genes via NanoString™, and protein content from conditioned media collected at days 1, 3 and 6 was analyzed via enzyme linked immunosorbent assays.
Results and Discussion
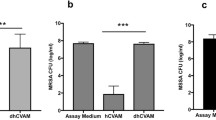
Gene expression analysis showed that Soluble Factors promoted significant upregulation of pro-inflammatory marker IL1B on day 1 yet downregulation of TNF on day 6 compared to the M1 macrophage control. In contrast, intact hCVAM, which includes both extracellular matrix, viable cells, and soluble factors, promoted downregulation of pro-inflammatory markers TNF, CCL5 and CCR7 on day 1 and endothelial receptor TIE1 on day 6, and upregulation of the anti-inflammatory marker IL10 on day 6 compared to the M1 Control. Other genes related to inflammation and angiogenesis (MMP9, VEGF, SPP1, TGFB1, etc.) were differentially regulated between the Soluble Factors and intact hCVAM groups at both time points, though they were not expressed at significantly different levels compared to the M1 Control. Interestingly, Soluble Factors promoted increased secretion of the pro-inflammatory cytokine tumor necrosis factor-α (TNF-α), while direct contact with hCVAM inhibited secretion of TNF, relative to the M1 Control. Both Soluble Factors and intact hCVAM inhibited secretion of MMP9 and VEGF, pro-inflammatory proteins that are critical for angiogenesis and remodeling, compared to the M1 Control, with intact hCVAM having a stronger effect.
Conclusions
In a simulated pro-inflammatory environment, intact hCVAM has distinct anti-inflammatory effects on primary human macrophages, and direct macrophage contact with intact hCVAM is required for these effects. These findings are important for the design of next generation immunomodulatory biomaterials for wound repair and regenerative medicine that may include living cells, soluble factors, or a controlled drug delivery system.




Similar content being viewed by others
Abbreviations
- hAM:
-
Human amniotic membrane
- ANOVA:
-
Analysis of variance
- CCL5:
-
Chemokine (C–C motif) ligand 5
- cRPMI:
-
Complete RPMI culture medium
- cRPMI-M1:
-
Complete RPMI culture medium supplemented with M1-stimulating cytokines
- ECM:
-
Extracellular matrix
- EGF:
-
Epidermal growth factor
- ELISA:
-
Enzyme-linked immunosorbent assay
- ERCC:
-
External RNA Control Consortium
- hCVAM:
-
Human cryopreserved amniotic membrane
- IFNG:
-
Interferon-γ
- IL4:
-
Interleukin-4
- IL8:
-
Interleukin-8
- IL10:
-
Interleukin-10
- IL1A:
-
Interleukin-1α
- IL1B:
-
Interleukin-1β
- LPS:
-
Lipopolysaccharide
- LMAM:
-
Living micronized amniotic membrane
- MCSF:
-
Macrophage colony stimulating factor
- MMP9:
-
Matrix metalloproteinase-9
- MSCs:
-
Mesenchymal stem cells
- PBMCs:
-
Peripheral blood mononuclear cells
- PBS:
-
Phosphate buffered saline
- PDGFB:
-
Platelet derived growth factor
- PGE2:
-
Prostaglandin E2
- SEM :
-
Standard error of mean
- TGFB1:
-
Transforming growth factor-β1
- TNF-α :
-
Tumor necrosis factor-α
- VEGF:
-
Vascular endothelial growth factor
References
Abraham, A., et al. Machine learning for neuroimaging with scikit-learn. Front. Neuroinform. 8:14, 2014.
Anderson, J. M., A. Rodriguez, and D. T. Chang. Foreign body reaction to biomaterials. Semin. Immunol. 20:86–100, 2008.
Arnold, L., et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 204:1057–1069, 2007.
Ashcroft, G. S., et al. Tumor necrosis factor-alpha (TNF-α) is a therapeutic target for impaired cutaneous wound healing. Wound Repair Regen. 20:38–49, 2012.
Badylak, S. F., J. E. Valentin, A. K. Ravindra, G. P. McCabe, and A. N. Stewart-Akers. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng. A 14:1835–1842, 2008.
Baluk, P., et al. TNF-α drives remodeling of blood vessels and lymphatics in sustained airway inflammation in mice. J. Clin. Investig. 119:2954–2964, 2009.
Benoit, M., B. Desnues, and J.-L. Mege. Macrophage polarization in bacterial infections. J. Immunol. 181:3733, 2008.
Berger, M. L., M. Mamdani, D. Atkins, and M. L. Johnson. Good research practices for comparative effectiveness research: defining, reporting and interpreting nonrandomized studies of treatment effects using secondary data sources: the ISPOR Good Research Practices for Retrospective Database Analysis Task Force Report—Part I. Value Health 12:1044–1052, 2009.
Brown, B. N., J. E. Valentin, A. M. Stewart-Akers, G. P. McCabe, and S. F. Badylak. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials 30:1482–1491, 2009.
Cooke, M., et al. Comparison of cryopreserved amniotic membrane and umbilical cord tissue with dehydrated amniotic membrane/chorion tissue. J. Wound Care 23:465–476, 2014.
Davis, J. S., et al. The use of skin grafts in the ambulatory treatment of ulcers: report of fifty cases. JAMA LXIV:558–560, 1915.
Duan-Arnold, Y., et al. Retention of endogenous viable cells enhances the anti-inflammatory activity of cryopreserved amnion. Adv. Wound Care (New Rochelle) 4:523–533, 2015.
Ferraro, N. M., W. Dampier, M. S. Weingarten, and K. L. Spiller. Deconvolution of heterogeneous wound tissue samples into relative macrophage phenotype composition via models based on gene expression. Integr. Biol. (Camb.) 9:328–338, 2017.
Gibbons, G. W. Grafix, a cryopreserved placental membrane, for the treatment of chronic/stalled wounds. Adv. Wound Care (New Rochelle) 4:534–544, 2015.
Guo, X., et al. Modulation of cell attachment, proliferation, and angiogenesis by decellularized, dehydrated human amniotic membrane in in vitro models. Wounds 29:28–38, 2017.
Hao, Y., D. H. Ma, D. G. Hwang, W. S. Kim, and F. Zhang. Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea 19:348–352, 2000.
Hopkinson, A., et al. Optimization of amniotic membrane (AM) denuding for tissue engineering. Tissue Eng. C 14:371–381, 2008.
Huang, G., et al. Accelerated expansion of epidermal keratinocyte and improved dermal reconstruction achieved by engineered amniotic membrane. Cell Transplant. 22:1831–1844, 2013.
Jansky, L., P. Reymanova, and J. Kopecky. Dynamics of cytokine production in human peripheral blood mononuclear cells stimulated by LPS or infected by Borrelia. Physiol. Res. 52:593–598, 2003.
Johnson, E. L., J. T. Marshall, and G. M. Michael. A comparative outcomes analysis evaluating clinical effectiveness in two different human placental membrane products for wound management. Wound Repair Regen. 2017. doi:10.1111/wrr.12503.
Laurent, R., A. Nallet, L. Obert, L. Nicod, and F. Gindraux. Storage and qualification of viable intact human amniotic graft and technology transfer to a tissue bank. Cell Tissue Bank. 15:267–275, 2014.
Lavery, L. A., et al. The efficacy and safety of Grafix((R)) for the treatment of chronic diabetic foot ulcers: results of a multi-centre, controlled, randomised, blinded, clinical trial. Int. Wound J. 11:554–560, 2014.
Lavin, Y., et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 159:1312–1326, 2014.
Law, C. W., Y. Chen, W. Shi, and G. K. Smyth. Voom: precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 15:R29, 2014.
Leibovich, S. J., and R. Ross. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am. J. Pathol. 78:71–100, 1975.
Litwiniuk, M., and T. Grzela. Amniotic membrane: new concepts for an old dressing. Wound Repair Regen. 22:451–456, 2014.
Liu, D., H. Xiong, P. Ning, J. Chen, and W. Lan. In: 2010 3rd International Conference on Biomedical Engineering and Informatics, Vol. 4, pp. 1633–1635, 2010.
Lurier, E. B., et al. Transcriptome analysis of IL-10-stimulated (M2c) macrophages by next-generation sequencing. Immunobiology 222(7):847–856, 2017.
Magatti, M., et al. Human amnion favours tissue repair by inducing the M1-to-M2 switch and enhancing M2 macrophage features. J. Tissue Eng. Regen. Med. 2016. doi:10.1002/term.2193.
Markova, A., and E. N. Mostow. US skin disease assessment: ulcer and wound care. Dermatol. Clin. 30:107–111, ix, 2012.
Mirza, R., L. A. DiPietro, and T. J. Koh. Selective and specific macrophage ablation is detrimental to wound healing in mice. Am. J. Pathol. 175:2454–2462, 2009.
Mirza, R. E., M. M. Fang, W. J. Ennis, and T. J. Koh. Blocking interleukin-1β induces a healing-associated wound macrophage phenotype and improves healing in Type 2 diabetes. Diabetes 62:2579–2587, 2013.
Mirza, R. E., M. M. Fang, E. M. Weinheimer-Haus, W. J. Ennis, and T. J. Koh. Sustained inflammasome activity in macrophages impairs wound healing in Type 2 diabetic humans and mice. Diabetes 63:1103–1114, 2014.
Mirza, R., and T. J. Koh. Dysregulation of monocyte/macrophage phenotype in wounds of diabetic mice. Cytokine 56:256–264, 2011.
Mosser, D. M., and J. P. Edwards. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8:958–969, 2008.
Nassiri, S., I. Zakeri, M. S. Weingarten, and K. L. Spiller. Relative expression of proinflammatory and antiinflammatory genes reveals differences between healing and nonhealing human chronic diabetic foot ulcers. J. Investig. Dermatol. 135:1700–1703, 2015.
Niknejad, H., G. Paeini-Vayghan, F. A. Tehrani, M. Khayat-Khoei, and H. Peirovi. Side dependent effects of the human amnion on angiogenesis. Placenta 34:340–345, 2013.
Parolini, O., and M. Caruso. Review: preclinical studies on placenta-derived cells and amniotic membrane: an update. Placenta 32(Suppl 2):S186–S195, 2011.
Regulski, M., et al. A retrospective analysis of a human cellular repair matrix for the treatment of chronic wounds. Ostomy Wound Manag. 59:38–43, 2013.
Roh, J. D., et al. Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proc. Natl Acad. Sci. USA 107:4669–4674, 2010.
Sainson, R. C. A., et al. TNF primes endothelial cells for angiogenic sprouting by inducing a tip cell phenotype. Blood 111:4997–5007, 2008.
Seabold, S., and P. Josef. Statsmodels: econometric and statistical modeling with Python. In: Proceedings of the 9th Python in Science Conference, pp 57–61, 2010.
Sen, C. K., et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 17:763–771, 2009.
Sindrilaru, A., et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J. Clin. Investig. 121:985–997, 2011.
Singh, S., et al. Unbiased analysis of the impact of micropatterned biomaterials on macrophage behavior provides insights beyond predefined polarization states. ACS Biomater. Sci. Eng. 2017. doi:10.1021/acsbiomaterials.7b00104.
Spiller, K. L., and T. J. Koh. Macrophage-based therapeutic strategies in regenerative medicine. Adv. Drug Deliv. Rev. 2017. doi:10.1016/j.addr.2017.05.010.
Spiller, K. L., et al. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials 35:4477–4488, 2014.
Talmi, Y. P., L. Sigler, E. Inge, Y. Finkelstein, and Y. Zohar. Antibacterial properties of human amniotic membranes. Placenta 12:285–288, 1991.
Tseng, S. C., D. Q. Li, and X. Ma. Suppression of transforming growth factor-beta isoforms, TGF-beta receptor type II, and myofibroblast differentiation in cultured human corneal and limbal fibroblasts by amniotic membrane matrix. J. Cell. Physiol. 179:325–335, 1999.
van Putten, S. M., D. T. A. Ploeger, E. R. Popa, and R. A. Bank. Macrophage phenotypes in the collagen-induced foreign body reaction in rats. Acta Biomater. 9:6502–6510, 2013.
Wetzler, C., H. Kämpfer, B. Stallmeyer, J. Pfeilschifter, and S. Frank. Large and sustained induction of chemokines during impaired wound healing in the genetically diabetic mouse: prolonged persistence of neutrophils and macrophages during the late phase of repair. J. Investig. Dermatol. 115:245–253, 2000.
Willenborg, S., et al. CCR2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood 120:613–625, 2012.
Wilshaw, S. P., J. N. Kearney, J. Fisher, and E. Ingham. Production of an acellular amniotic membrane matrix for use in tissue engineering. Tissue Eng. 12:2117–2129, 2006.
Wilshaw, S. P., J. Kearney, J. Fisher, and E. Ingham. Biocompatibility and potential of acellular human amniotic membrane to support the attachment and proliferation of allogeneic cells. Tissue Eng. A 14:463–472, 2008.
Witherel, C. E., P. L. Graney, D. O. Freytes, M. S. Weingarten, and K. L. Spiller. Response of human macrophages to wound matrices in vitro. Wound Repair Regen. 24:514–524, 2016.
Wolbank, S., et al. Impact of human amniotic membrane preparation on release of angiogenic factors. J. Tissue Eng. Regen. Med. 3:651–654, 2009.
Wynn, T. A., and L. Barron. Macrophages: master regulators of inflammation and fibrosis. Semin. Liver Dis. 30:245–257, 2010.
Xue, J., et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 40:274–288, 2014.
Zheng, Y., et al. Topical administration of cryopreserved living micronized amnion accelerates wound healing in diabetic mice by modulating local microenvironment. Biomaterials 113:56–67, 2017.
Acknowledgments
The authors would like to thank Yi Arnold-Duan and Matthew Moorman (Osiris Therapeutics, Inc.) for their helpful discussions and technical advice in handling hCVAM. This work was sponsored in part by Osiris Therapeutics, Inc., and by NHLBI Grant Number R01 HL130037 to KLS. CEW is grateful for the US Department of Education Graduate Assistance in Areas of National Need (GAANN) Interdisciplinary Collaboration and Research Enterprise (iCARE) Fellowship.
Conflict of interest
KLS discloses a potential conflict of interest: this study was funded in large part by Osiris Therapeutics, Inc. The study was designed by KLS and CEW, with some input from Osiris with respect to the potential impact of different experiments. Employees from Osiris had no part in interpretation of the study’s results. CEW, TY, MC, and WD declare that they have no conflicts of interest.
Ethical Approval
De-identified hCVAM samples were provided by Osiris Therapeutics as commercially available materials. De-identified human monocytes were purchased from the University of Pennsylvania Human Immunology Core. As such both human materials are exempt from review by the Institutional Review Board. No animal experiments were conducted for this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Alyssa Panitch oversaw the review of this article.
Kara Spiller is an Assistant Professor in Drexel University’s School of Biomedical Engineering, Science, and Health Systems. Dr. Spiller received Bachelor’s and Master’s Degrees in Biomedical Engineering from Drexel in 2007. As an NSF Graduate Research Fellow, she conducted her doctoral research in the design of semi-degradable hydrogels for the repair of articular cartilage in the Biomaterials and Drug Delivery Laboratory at Drexel and in the Shanghai Key Tissue Engineering Laboratory of Shanghai Jiao Tong University. After completing her Ph.D. in 2010, she conducted research in the design of scaffolds for bone tissue engineering on a Fulbright Fellowship in the Biomaterials, Biodegradables, and Biomimetics (the 3Bs) Research Group at the University of Minho in Guimarães, Portugal. She then conducted postdoctoral studies towards the development of immunomodulatory biomaterials for bone regeneration in the Laboratory for Stem Cells and Tissue Engineering at Columbia University, before joining the Faculty of Drexel in 2013. Her research is funded by grants from the National Science Foundation and the NIH, as well as grants from private foundations and industry. Her research interests include the role of inflammation in regenerative medicine, the design of immunomodulatory biomaterials, and international engineering education.

This article is part of the 2017 CMBE Young Innovators special issue.
Electronic supplementary material
Below is the link to the electronic supplementary material. Figure S1 Principal component analysis of principal component 1 (PC1) vs. principal component 2 (PC2), which account for 42.5 and 15% of the variance, respectively. Each treatment is represented by a color, M1 Control: grey, Direct Contact: blue, Soluble Factors: gold, while each donor is represented by a different symbol. Filled markers represent day 1 samples while outlined (no-fill) markers represent day 6 samples. Figure S2 NanoString gene expression analysis of all additional genes that were not differentially expressed. Data are represented as Log2(Value/M1 Control) and as the mean of all experimental replicates (n = 4–9) ± standard error of the mean (SEM). A dotted line at a fold change of 1.0 (or 0 on graphs of Log2-transformed data of values normalized to the M1 Control) on each individual gene represents no change vs. the M1 Control. Figure S3 Differentially expressed gene, IL1B, via mixed-effects regression with treatment and time held as fixed effects and donor as a random effect with ***p < 0.0001. Treatment group with a decreasing slope over time indicates a downregulation over time.
Rights and permissions
About this article
Cite this article
Witherel, C.E., Yu, T., Concannon, M. et al. Immunomodulatory Effects of Human Cryopreserved Viable Amniotic Membrane in a Pro-Inflammatory Environment In Vitro . Cel. Mol. Bioeng. 10, 451–462 (2017). https://doi.org/10.1007/s12195-017-0494-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-017-0494-7