Abstract
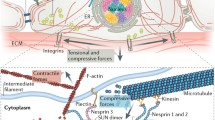
The cell nucleus is becoming increasingly recognized as a mechanosensitive organelle. Most research on nuclear mechanosignaling focuses on the nuclear lamina and coupled actin structures. In this commentary, we discuss the possibility that the nuclear membrane senses and transduces mechanical signals similar to the plasma membrane. We briefly summarize possible (i) pathophysiological sources of nuclear membrane tension, (ii) features that render nuclear membranes particularly suited for mechanotransduction, and (iii) molecular sensing mechanisms.


Similar content being viewed by others
References
Alam, S., D. B. Lovett, R. B. Dickinson, K. J. Roux, and T.P. Lele. Nuclear forces and cell mechanosensing. 1st ed. Prog. Mol. Biol. Transl. Sci. http://dx.doi.org/10.1016/B978-0-12-394624-9.00008-7, 2014.
Bazán, N. G. Effects of ischemia and electroconvulsive shock on free fatty acid pool in the brain. Biochim. Biophys. Acta 218:1–10, 1970.
Berghe, T. V., A. Linkermann, S. Jouan-Lanhouet, H. Walczak, and P. Vandenabeele. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat. Rev. Mol. Cell Biol. 15:135–147. http://www.nature.com/doifinder/10.1038/nrm3737, 2014.
Bigay, J., and B. Antonny. Curvature, lipid packing, and electrostatics of membrane organelles: defining cellular territories in determining specificity. Dev. Cell 23:886–895, 2012.
Boguslavsky, V., M. Rebecchi, A. J. Morris, D. Y. Jhon, S. G. Rhee, and S. McLaughlin. Effect of monolayer surface pressure on the activities of phosphoinositide-specific phospholipase C-beta 1, -gamma 1, and -delta 1. Biochemistry 33:3032–3037. http://www.ncbi.nlm.nih.gov/pubmed/8130216, 1994.
Dahl, K. N., S. M. Kahn, K. L. Wilson, and D. E. Discher. The nuclear envelope lamina network has elasticity and a compressibility limit suggestive of a molecular shock absorber. J. Cell Sci. 117:4779–86. http://www.ncbi.nlm.nih.gov/pubmed/15331638, 2004.
Davidson, P. M., J. Sliz, P. Isermann, C. Denais, and J. Lammerding. Design of a microfluidic device to quantify dynamic intra-nuclear deformation during cell migration through confining environments. Integr. Biol. (Camb). 7:1534–1546. http://www.ncbi.nlm.nih.gov/pubmed/26549481, 2015.
Diz-Muñoz, A., D. a Fletcher, and O. D. Weiner. Use the force: membrane tension as an organizer of cell shape and motility. Trends Cell Biol. 23:47–53. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3558607&tool=pmcentrez&rendertype=abstract, 2013.
Dodes Traian, M. M., F. L. González Flecha, and V. Levi. Imaging lipid lateral organization in membranes with C-laurdan in a confocal microscope. J. Lipid Res. 53:609–616, 2012.
Enyedi, B., S. Kala, T. Nikolich-Zugich, and P. Niethammer. Tissue damage detection by osmotic surveillance. Nat. Cell Biol. http://www.ncbi.nlm.nih.gov/pubmed/23934216, 2013.
Enyedi, B., and P. Niethammer. Mechanisms of epithelial wound detection. Trends Cell Biol. 25:398–407, 2015.
Fedorchak, G. R., A. Kaminski, and J. Lammerding. Cellular mechanosensing: getting to the nucleus of it all. Prog. Biophys. Mol. Biol. http://linkinghub.elsevier.com/retrieve/pii/S0079610714000510, 2014.
Fidorra, J., T. Mielke, J. Booz, and L. E. Feinendegen. Cellular and nuclear volume of human cells during the cell cycle. Radiat. Environ. Biophys. 19:205–214, 1981.
Finan, J. D., and F. Guilak. The effects of osmotic stress on the structure and function of the cell nucleus. J. Cell. Biochem. 109:460–467. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3616882&tool=pmcentrez&rendertype=abstract, 2010.
Finan, J. D., K.J. Chalut, A. Wax, and F. Guilak. Nonlinear osmotic properties of the cell nucleus. Ann. Biomed. Eng. 37:477–491. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2749482&tool=pmcentrez&rendertype=abstract, 2009.
Friedl, P., K. Wolf, and J. Lammerding. Nuclear mechanics during cell migration. Curr. Opin. Cell Biol. 23:55–64. http://linkinghub.elsevier.com/retrieve/pii/S0955067410001869, 2011.
Gault, W. J., B. Enyedi, and P. Niethammer. Osmotic surveillance mediates rapid wound closure through nucleotide release. J. Cell Biol. 207:767–782, 2014.
Gauthier, N. C., M. A. Fardin, P. Roca-Cusachs, and M. P. Sheetz. Temporary increase in plasma membrane tension coordinates the activation of exocytosis and contraction during cell spreading. Proc. Natl. Acad. Sci. USA 108:14467–14472, 2011.
Gauthier, N. C., T. A. Masters, and M. P. Sheetz. Mechanical feedback between membrane tension and dynamics. Trends Cell Biol. 22:527–535, 2012.
Guilluy, C., and K. Burridge. Nuclear mechanotransduction: forcing the nucleus to respond. Nucleus 6:19–22, 2015.
Guilluy, C. et al. Isolated nuclei adapt to force and reveal a mechanotransduction pathway in the nucleus. Nat. Cell Biol. 16:376–381. http://www.ncbi.nlm.nih.gov/pubmed/24609268, 2014.
Hamill, O. P., and B. Martinac. Molecular basis of mechanotransduction in living cells. Physiol. Rev. 81:685–740, 2001.
Hategan, A., R. Law, S. Kahn, and D. E. Discher. Adhesively-tensed cell membranes: lysis kinetics and atomic force microscopy probing. Biophys. 85:2746–2759. http://dx.doi.org/10.1016/S0006-3495(03)74697-9, 2003.
Irianto, J. et al. Osmotic challenge drives rapid and reversible chromatin condensation in chondrocytes. Biophys. J. Biophys. Soc. 104:759–769. http://dx.doi.org/10.1016/j.bpj.2013.01.006, 2013.
Itano, N., S. Okamoto, D. Zhang, S. A Lipton, and E. Ruoslahti. Cell spreading controls endoplasmic and nuclear calcium: a physical gene regulation pathway from the cell surface to the nucleus. Proc. Natl. Acad. Sci. USA. 100:5181–5186. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=154319&tool=pmcentrez&rendertype=abstract, 2003.
Janmey, P. A., and P. K. J. Kinnunen. Biophysical properties of lipids and dynamic membranes. Trends Cell Biol. 16:538–546, 2006.
Lammerding, J., K. N. Dahl, D. E. Discher, and R. D. Kamm. Nuclear mechanics and methods. Methods Cell Biol. 83:269–294. http://www.ncbi.nlm.nih.gov/pubmed/17613312, 2007.
Lehtonen, J. Y., and P. K. Kinnunen. Phospholipase A2 as a mechanosensor. Biophys. J. 68:1888–1894. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1282092&tool=pmcentrez&rendertype=abstract, 1995.
Liang, D., S. Bhatta, V. Gerzanich, and J. M. Simard. Cytotoxic edema: mechanisms of pathological cell swelling. Neurosurg. Focus 22:E2. http://www.ncbi.nlm.nih.gov/pubmed/1761323, 2007.
Majno, G., and I. Joris. Apoptosis, oncosis, and necrosis. An overview of cell death. Am. J. Pathol. 146:3–15. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1870771&tool=pmcentrez&rendertype=abstract, 1995.
Niethammer, P. Healed by our inner fish? Oncotarget 6:15732–15733, 2015.
Osmanagic-myers, S., T. Dechat, and R. Foisner. Lamins at the crossroads of mechanosignaling. Genes Dev. 29:225–237, 2015.
Peters-Golden, M., and T. G. Brock. Intracellular compartmentalization of leukotriene synthesis: unexpected nuclear secrets. FEBS Lett. 487:323–326, 2001.
Prat, A. G., and H. F. Cantiello. Nuclear ion channel activity is regulated by actin filaments. Am. J. Physiol. 270:C1532–C1543, 1996.
Prescott, D. M. Relation between cell growth and cell division. III. Changes in nuclear volume and growth rate and prevention of cell division in Amoeba proteus resulting from cytoplasmic amputations. Exp. Cell Res. 11:94–98, 1956.
Raffy, S., and J. Teissié. Control of lipid membrane stability by cholesterol content. Biophys. J. 76:2072–2080, 1999.
Redondo-Morata, L., M. I. Giannotti, and F. Sanz. Influence of cholesterol on the phase transition of lipid bilayers: a temperature-controlled force spectroscopy study. Langmuir 28:12851–12860, 2012.
Roca-Cusachs, P., J. Alcaraz, R. Sunyer, J. Samitier, R. Farré, and D. Navajas. Micropatterning of single endothelial cell shape reveals a tight coupling between nuclear volume in G1 and proliferation. Biophys. J. 94:4984–4995, 2008.
Sezgin, E., T. Sadowski, and K. Simons. Measuring lipid packing of model and cellular membranes with environment sensitive probes. Langmuir 30:8160–8166, 2014.
Souvignet, C., J. M. Pelosin, S. Daniel, E. M. Chambaz, S. Ransac, and R. Verger. Activation of protein kinase C in lipid monolayers. J. Biol. Chem. 266:40–44, 1991.
von Moltke, J. et al. Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature 490:107–111. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3465483&tool=pmcentrez&rendertype=abstract, 2012.
Zhang, Y.-L., J. A. Frangos, and M. Chachisvilis. Laurdan fluorescence senses mechanical strain in the lipid bilayer membrane. Biochem. Biophys. Res. Commun. 347:838–841, 2006.
Acknowledgments
The authors would like to thank Kris Noel Dahl for helpful discussions. The authors are supported by the National Institutes of Health Grant GM099970 to P.N.
Conflict of interest
Balázs Enyedi and Philipp Niethammer declare that they have no conflicts of interest.
Ethical standards
No human studies were carried out by the authors for this article. No animal studies were carried out by the authors for this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor G.W. Gant Luxton oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Enyedi, B., Niethammer, P. A Case for the Nuclear Membrane as a Mechanotransducer. Cel. Mol. Bioeng. 9, 247–251 (2016). https://doi.org/10.1007/s12195-016-0430-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-016-0430-2