Abstract
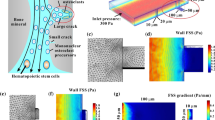
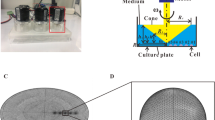
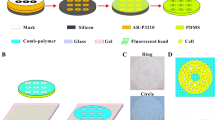
Osteoclasts are large multinucleate cells originating from the fusion of monocytes that are differentiated from hematopoietic stem cells. Although activated osteoclasts preferentially move to the area of microcrack by chemotaxis, whether such mechanical cues as fluid shear stress (FSS) regulate the migration of osteoclasts remains unknown. This study focuses on the effect of FSS on the migration of RAW264.7 monocytes and differentiated osteoclasts, as well as the roles of calcium signaling pathways in cell migration behaviors. We study five calcium signaling pathways, namely, mechanosensitive cation-selective channels (MSCC), phospholipase C, endoplasmic reticulum (ER), adenosine triphosphate, and extracellular calcium. Results show that FSS induces the migration of RAW264.7 cells along flow direction, and the directionality, alignment along the flow direction, and speed of cells are significantly enhanced with the increase in FSS levels. Blocking the pathways of MSCC, ER, or extracellular calcium significantly reduces the migration of RAW264.7 cells along the flow direction. However, the inhibition of calcium signaling pathways does not affect the migration behaviors after inducing RAW264.7 cells for 4 or 8 days with the conditioned medium, Therefore, this study indicates that both undifferentiated monocytes and differentiated osteoclasts tend to migrate along flow direction, furthermore the FSS-induced directional migration of the monocytes is regulated by calcium signaling pathways, but that of differentiated osteoclasts is unaffected.






Similar content being viewed by others
References
Al-Dujaili, S. A., E. Lau, et al. Apoptotic osteocytes regulate osteoclast precursor recruitment and differentiation in vitro. J. Cell Biochem. 112(9):2412–2423, 2011.
Baron, R., L. Neff, et al. Kinetic and cytochemical identification of osteoclast precursors and their differentiation into multinucleated osteoclasts. Am. J. Pathol. 122(2):363–378, 1986.
Baroukh, B., M. Cherruau, et al. Osteoclasts differentiate from resident precursors in an in vivo model of synchronized resorption: a temporal and spatial study in rats. Bone 27(5):627–634, 2000.
Bentolila, V., T. M. Boyce, et al. Intracortical remodeling in adult rat long bones after fatigue loading. Bone 23(3):275–281, 1998.
Boudot, C., Z. Saidak, et al. Implication of the calcium sensing receptor and the phosphoinositide 3-kinase/Akt pathway in the extracellular calcium-mediated migration of RAW 264.7 osteoclast precursor cells. Bone 46(5):1416–1423, 2010.
Bruck, H. A., S. R. McNeill, et al. Digital image correlation using Newton–Raphson method of partial-differential correction. Exp. Mech. 29(3):261–267, 1989.
Brundage, R. A., K. E. Fogarty, et al. Calcium gradients underlying polarization and chemotaxis of eosinophils. Science 254(5032):703–706, 1991.
Cardoso, L., B. C. Herman, et al. Osteocyte apoptosis controls activation of intracortical resorption in response to bone fatigue. J. Bone Miner. Res. 24:597–605, 2008.
Chandran, P., A. Sasidharan, et al. Highly biocompatible TiO(2):Gd(3)(+) nano-contrast agent with enhanced longitudinal relaxivity for targeted cancer imaging. Nanoscale 3(10):4150–4161, 2011.
Eriksen, E. F. Cellular mechanisms of bone remodeling. Rev. Endocr. Metab. Disord. 11(4):219–227, 2010.
Fahlgren, A., M. P. G. Bostrom, et al. Fluid pressure and flow as a cause of bone resorption. Acta Orthop. 81(4):508–516, 2010.
Gardinier, J. D., C. W. Townend, et al. In situ permeability measurement of the mammalian lacunar-canalicular system. Bone 46(4):1075–1081, 2010.
Hadjidakis, D. J., and I. I. Androulakis. Bone remodeling. Ann. NY Acad. Sci. 1092:385–396, 2006.
He, S., Y. Su, et al. Some basic questions on mechanosensing in cell-substrate interaction. J. Mech. Phys. Solids 70:116–135, 2014.
Herman, B. C., L. Cardoso, et al. Activation of bone remodeling after fatigue: differential response to linear microcracks and diffuse damage. Bone 47(4):766–772, 2010.
Ishii, M., J. Kikuta, et al. Chemorepulsion by blood S1P regulates osteoclast precursor mobilization and bone remodeling in vivo. J. Exp. Med. 207(13):2793–2798, 2010.
Johansson, L., U. Edlund, et al. Bone resorption induced by fluid flow. J. Biomech. Eng. 131(9):094505, 2009.
Kennedy, O. D., B. C. Herman, et al. Activation of resorption in fatigue-loaded bone involves both apoptosis and active pro-osteoclastogenic signaling by distinct osteocyte populations. Bone 50(5):1115–1122, 2012.
Kikuta, J., S. Kawamura, et al. Sphingosine-1-phosphate-mediated osteoclast precursor monocyte migration is a critical point of control in antibone-resorptive action of active vitamin D. Proc. Natl. Acad. Sci. USA 110(17):7009–7013, 2013.
Kim, S. Y., S. H. Park, et al. Mechanical stimulation and the presence of neighboring cells greatly affect migration of human mesenchymal stem cells. Biotechnol. Lett. 35(11):1817–1822, 2013.
Koizumi, K., Y. Saitoh, et al. Role of CX3CL1/fractalkine in osteoclast differentiation and bone resorption. J. Immunol. 183(12):7825–7831, 2009.
Kong, D., B. H. Ji, et al. Stabilizing to disruptive transition of focal adhesion response to mechanical forces. J. Biomech. 43(13):2524–2529, 2010.
Li, P., M. Hu, et al. Fluid flow-induced calcium response in early or late differentiated osteoclasts. Ann. Biomed. Eng. 40(9):1874–1883, 2012.
Li, P., C. Liu, et al. Fluid flow-induced calcium response in osteoclasts: signaling pathways. Ann. Biomed. Eng. 42(6):1250–1260, 2014.
Liu, Y., L. Li, et al. Effects of fluid shear stress on bone resorption in rat osteoclasts. J. Biomech. Eng. 24(3):544–548, 2007.
Masuyama, R., J. Vriens, et al. TRPV4-mediated calcium influx regulates terminal differentiation of osteoclasts. Cell Metab. 8(3):257–265, 2008.
Polacheck, W. J., J. L. Charest, et al. Interstitial flow influences direction of tumor cell migration through competing mechanisms. Proc. Natl. Acad. Sci. USA 108(27):11115–11120, 2011.
Schwab, A., F. Finsterwalder, et al. Intracellular Ca2+ distribution in migrating transformed epithelial cells. Pflugers Arch. 434(1):70–76, 1997.
Sheikh, S., G. E. Rainger, et al. Exposure to fluid shear stress modulates the ability of endothelial cells to recruit neutrophils in response to tumor necrosis factor-alpha: a basis for local variations in vascular sensitivity to inflammation. Blood 102(8):2828–2834, 2003.
Shiu, Y. T., S. Li, et al. Rho mediates the shear-enhancement of endothelial cell migration and traction force generation. Biophys. J. 86(4):2558–2565, 2004.
Teitelbaum, S. L. Bone resorption by osteoclasts. Science 289(5484):1504–1508, 2000.
Thompson, W. R., C. T. Rubin, et al. Mechanical regulation of signaling pathways in bone. Gene 503(2):179–193, 2012.
Tsuzuki, T., K. Okabe, et al. Osmotic membrane stretch increases cytosolic Ca(2+) and inhibits bone resorption activity in rat osteoclasts. Jpn. J. Physiol. 50(1):67–76, 2000.
Waldorff, E. I., K. B. Christenson, et al. Microdamage repair and remodeling requires mechanical loading. J. Bone Miner. Res. 25(4):734–745, 2010.
Wang, H., W. Sun, et al. Polycystin-1 mediates mechanical strain-induced osteoblastic mechanoresponses via potentiation of intracellular calcium and Akt/beta-catenin pathway. PLoS One 9(3):e91730, 2014.
Wang, L., T. Ye, et al. Repair of microdamage in osteonal cortical bone adjacent to bone screw. PLoS One 9(2):e89343, 2014.
Wei, C., X. Wang, et al. Calcium flickers steer cell migration. Nature 457(7231):901–905, 2009.
Weinbaum, S., S. C. Cowin, et al. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J. Biomech. 27(3):339–360, 1994.
Wheal, B. D., R. J. Beach, et al. Subcellular elevation of cytosolic free calcium is required for osteoclast migration. J. Bone Miner. Res. 29(3):725–734, 2014.
Wiebe, S. H., S. M. Sims, et al. Calcium signalling via multiple P2 purinoceptor subtypes in rat osteoclasts. Cell. Physiol. Biochem. 9(6):323–337, 1999.
Xia, S. L., and J. Ferrier. Calcium signal induced by mechanical perturbation of osteoclasts. J. Cell. Physiol. 163(3):493–501, 1995.
Xia, S. L., and J. Ferrier. Localized calcium signaling in multinucleated osteoclasts. J. Cell. Physiol. 167(1):148–155, 1996.
Zhong, Y., and B. Ji. How do cells produce and regulate the driving force in the process of migration? Eur. Phys. J. Spec. Top. 223(7):1373–1390, 2014.
Acknowledgments
This work was supported by the National Natural Science Foundation of China [11372043 (BH), 11025208, 11372042, and 11221202 (BJ)] and the Fundamental Research Funds for the Central Universities [GZ2013015101 (BH)].
Conflict of interest
Chenglin Liu, Shuna Li, Baohua Ji, and Bo Huo declare that they have no conflicts of interest.
Ethical Standards
No human or animal studies were carried out by the authors for this article.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Associate Editor Daniel Fletcher oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Liu, C., Li, S., Ji, B. et al. Flow-Induced Migration of Osteoclasts and Regulations of Calcium Signaling Pathways. Cel. Mol. Bioeng. 8, 213–223 (2015). https://doi.org/10.1007/s12195-014-0372-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-014-0372-5