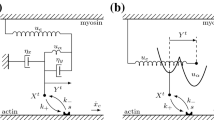
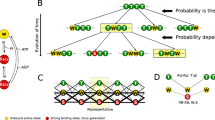
Abstract
Mechanochemical interactions between myosin and actin-troponin-tropomyosin (regulated thin filaments) underlie muscle contraction. Generally, the binding of a myosin molecule to a regulated thin filament facilitates the binding of nearby myosin; binding is cooperative. A simple yet accurate theoretical description of this cooperativity is lacking. From a general mechanochemical model that treats tropomyosin as a continuous beam, we show that cooperative interactions can be defined by two parameters: \(\mathcal{C}, \) which specifies the number of neighboring molecules affected by myosin binding, and δ, which specifies how regulation slows myosin’s binding rate to actin. We then propose two methods to derive differential equations describing cooperative ensembles of myosin interacting with regulated thin filaments: the weakly-correlated and the linear theory. The linear theory fits measurements of the speed of regulated thin filaments moving over a dense bed of myosin at low calcium, giving rapid and precise estimates of \(\mathcal{C}=11\pm 2\) and δ = 0.003 ± 0.002. The theory clarifies the relationship between microscopic measurements and macroscopic properties, serving as a step toward a complete multi-scale description of muscle contraction.




Similar content being viewed by others
References
Als-Nielson, J., and R. J. Birgeneau. Mean field theory, the Ginzburg criterion and marginal dimensionality of phase transitions. Am. J. Phys. 45:554–560, 1977.
Campbell, K. S. Interactions between connected half-sarcomeres produce emergent mechanical behavior in a mathematical model of muscle. PLoS Comp. Biol. 7:e1000560, 2009.
Craig, R., and W. Lehman. Crossbridge and tropomyosin positions observed in native, interacting thick and thin filaments. J. Mol. Biol. 311:1027–1036, 2001.
Debold, E. P., W. Saber, Y. Cheema, C. S. Bookwalter, K. M. Trybus, D. M. Warshaw, and P. J. VanBuren. Human actin mutations associated with hypertrophic and dilated cardiomyopathies demonstrate distinct thin filament regulatory properties in vitro. Mol. Cell. Cardiol. 48:286–292, 2010.
Duke, T. A. J. Molecular model of muscle contraction. Proc. Natl. Acad. Sci. 96:2770–2775, 1999.
Eisenberg, E., T. L. Hill, and Y.-D. Chen. Cross-bridge model of muscle contraction. Quantitative analysis. Biophys J. 29:195–227, 1980.
Finer, J. T., R. M. Simmons, and J. A. Spudich. Single myosin molecule mechanics: piconewton forces and nanometre steps. Nature 368:113–119, 1994.
Geeves, M. A., and S. S. Lehrer. Dynamics of the muscle thin filament regulatory switch: the size of the cooperative unit. Biophys. J. 67:273–282, 1994.
Geeves, M., H. Griffiths, S. Mijailovich, and D. A. Smith. Cooperative [Ca2+]-dependent regulation of the rate of myosin binding to actin: solution data and the tropomyosin chain model. Biophys. J. 100:2679–2687, 2011.
Greene, L., and E. Eisenberg. Cooperative binding of myosin subfragment-1 to the actin-troponin-tropomyosin complex. Proc. Natl. Acad. Sci. 77:2616–2620, 1980.
Guilford, W. H., D. E. Depuis, G. Kennedy, J. Wu, J. B. Patlak, and D. M. Warshaw. Smooth muscle and skeletal muscle myosin produce similar unitary forces and displacements in the laser trap. Biophys. J. 72:1006–1021, 1997.
Harris, D. E., and D. M. Warshaw. Smooth and skeletal muscle myosin both exhibit low duty cycles at zero load in vitro. J. Biol. Chem. 268:14764–14768, 1993.
Hill, T. L. Theoretical formalism for the sliding filament model of contraction of striated muscle. Part I. Prog. Biophys. Mol. Biol. 28:267–340, 1974.
Hill, T. L., E. Eisenberg, and L. Greene. Theoretical model for the cooperative equilibrium binding of myosin subfragment 1 to the actin-troponin-tropomyosin complex. Proc. Natl. Acad. Sci. 77:3186–3190, 1980.
Hill, T. L., E. Eisenberg, and J. M. Chalovich. Theoretical models for cooperative steady-state ATPase activity of myosin subfragment-1 on regulated actin. Biophys. J. 35:99–112, 1981.
Huxley, A. F. Muscle structure and theories of contraction. Prog. Biophys. Biophys. Chem. 7:255–318, 1957.
Ishijima, A., Y. Harada, H. Kojima, T. Funatsu, H. Higuchi, and T. Yanagida. Single-molecule analysis of the actomyosin motor using nano-manipulation. Biochem. Biophys. Res. Commun. 199:1057–1063, 1994.
Kad, N. M., J. B. Patlak, P. M. Fagnant, K. M. Trybus, and D. M. Warshaw. Mutation of a conserved glycine in the SH1-SH2 helix affects the load-dependent kinetics of myosin. Biophys. J. 92:1623–1631, 2007.
Kad, N. M., A. S. Rovner, P. M. Fagnant, P. B. Joel, G. G. Kennedy, J. B. Patlak, D. M. Warshaw, and K. M. Trybus. A mutant heterodimeric myosin with one inactive head generates maximal displacement. J. Cell. Biol. 162:481–488, 2003.
Kad, N. M., S. Kim, D. M. Warshaw, P. VanBuren, and J. E. Baker. Single myosin crosbridge interactions with actin filaments regulated by troponin-tropomyosin. Proc. Natl. Acad. Sci. 102:16990–16995, 2005.
Lacker, H. M. Cross-bridge dynamics in skeletal muscle: mathematical methods for determining the reaction rate and force-extension curves of cross-bridges from the macroscopic behavior of muscle. PhD Thesis, NYU, 1977.
Lacker, H. M., and C. S. Peskin. A mathematical method for the unique determination of cross-bridge properties from steady-state mechanical and energetic experiments on macroscopic muscle. Lect. Math. Life Sci. 16:121–153, 1986.
Liu, Y., M. Scolari, W. Im, and H. J. Woo. Protein-protein interactions in actin-myosin binding and structural effects of R405Q mutation: a molecular dynamics study. Prot. Struct. Funct. Bioinf. 64:156–166, 2006.
Lymn, R. W., and E. W. Taylor. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry 10:4617–4624, 1971.
McKillop, D. F. A., and M. A. Geeves. Regulation of the interaction between actin and myosin subfragment I: evidence for three states of the thin filament. Biophys. J. 65:693–701, 1993.
Mijailovich, S. M., X. Li, R. H. Griffiths, and M. A. Geeves. The Hill model for binding myosin S1 to regulated actin is not equivalent to the McKillop-Geeves model. J. Mol. Biol. 417:112–128, 2012.
Molloy, J. E., J. E. Burns, J. Kendrick-Jones, R. T. Tregear, and D. C. S. White. Movement and force produced by a single myosin head . Nature 378:209–212, 1995.
Orlova, A., and E. H. Egelman. Cooperative rigor binding of myosin to actin is a function of F-actin structure. J. Mol. Biol. 265:469–474, 1997.
Sich, N. M., T. J. O’Donnell, S. A. Coulter, O. A. John, M. S. Carter, C. R. Cremo, and J. E. Baker. Effects of actin-myosin kinetics on the calcium sensitivity of regulated thin filaments. J. Biol. Chem. 285:39150–39159, 2010.
Smith, D. A. Path-integral theory of an axially confined worm-like chain. J. Phys. A: Math. Gen. 34:4507–4523, 2001.
Smith, D. A., R. Maytum, and M. A. Geeves. Cooperative regulation of myosin-actin interactions by a continuous flexible chain I: actin-tropomyosin systems. Biophys. J. 84:3155–3167, 2003.
Steffen, W., and J. Sleep. Repriming the acomyosin crossbridge cycle Proc. Natl. Acad. Sci. 101:12904–12909, 2004.
Stoecker, U., I. A. Telley, E. Stüssi, and J. J. Denoth. A multisegmental cross-bridge kinetics model of the myofibril. J. Theor. Biol. 259:714–726, 2009.
Tanner, B. C. W., T. L. Daniel, and M. Regnier. Sarcomere lattice geometry influences the cooperative myosin binding in muscle. PLoS Comp. Biol. 3:e115, 2007.
Uyeda, T. Q. P., S. J. Kron, and J. A. Spudich. Myosin step size: estimation from slow sliding movement of actin over low densities of heavy meromyosin. J. Mol. Biol. 214:699–710, 1990.
Veigel, C., J. E. Molloy, S. Schmitz, and J. Kendrick-Jones. Load-dependent kinetics of force production by smooth muscle myosin measured with optical tweezers. Nat. Cell Biol. 5:980–986, 2003.
Walcott, S., and S. X. Sun. Hysteresis in cross-bridge models of muscle. Phys. Chem. Chem. Phys. 11:4871–4881, 2009.
Walcott, S., D. M. Warshaw, and E. P. Debold. Mechanical coupling between myosin molecules causes differences between ensemble and single molecule measurements. Biophys. J. 103:501–510, 2012.
Acknowledgments
The author is grateful to Dr. Neil M. Kad for critiquing an earlier version of the manuscript, to Dr. Edward P. Debold and Matthew A. Turner for their experimental efforts, and to Dr. Sean X. Sun and Dr. Sven Bachmann for useful discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Jung-Chi Liao and Henry Hess oversaw the review of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Walcott, S. A Differential Equation Model for Tropomyosin-induced Myosin Cooperativity Describes Myosin–Myosin Interactions at Low Calcium. Cel. Mol. Bioeng. 6, 13–25 (2013). https://doi.org/10.1007/s12195-012-0259-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-012-0259-2