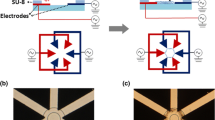
Abstract
Active transport by kinesin molecular motors has been used to assemble ring nanocomposites comprised of biotinylated microtubules and streptavidin-coated quantum dots. Here we studied the effects of two-dimensional surface confinement on ring self-assembly using substrates of microfluidic channels or periodic post arrays. Microfabricated devices were composed of gold–silicon oxide surfaces where the gold surfaces were functionalized with thiol-based self-assembled monolayers, which enabled selective adsorption of kinesin to silicon surfaces. Confinement of ring self-assembly within microfluidic channels was observed as a change in the distribution of ring diameters, specifically by placing an upper limit on the diameter capable of forming in the channels. Confining assembly using periodic post arrays where the edge-to-edge spacing was 2 μm resulted in a significantly smaller average diameter when compared against those formed in arrays with 5 and 10 μm spacing. Differences in diameters formed in 5 and 10 μm arrays were not observed. Observations of ring composite assembly along channel edges on the top surface, as well as around posts in the arrays confirm the fundamental role of active transport-induced mechanical strain in initiating the self-assembly process.







Similar content being viewed by others
References
Bachand, G. D., S. B. Rivera, A. K. Boal, J. Gaudioso, J. Liu, and B. C. Bunker. Assembly and transport of nanocrystal CdSe quantum dot nanocomposites using microtubules and kinesin motor proteins. Nano Lett. 4:817–821, 2004.
Bachand, M., A. M. Trent, B. C. Bunker, and G. D. Bachand. Physical factors affecting kinesin-based transport of synthetic nanoparticle cargo. J. Nanosci. Nanotechnol. 5:718–722, 2005.
Boal, A. K., G. D. Bachand, S. B. Rivera, and B. C. Bunker. Interactions between cargo-carrying biomolecular shuttles. Nanotechnology 17:349–354, 2006.
Cheng, L. J., M. T. Kao, E. Meyhofer, and L. J. Guo. Highly efficient guiding of microtubule transport with imprinted CYTOP nanotracks. Small 1:409–414, 2005.
Clemmens, J., H. Hess, J. Howard, and V. Vogel. Analysis of microtubule guidance in open microfabricated channels coated with the motor protein kinesin. Langmuir 19:1738–1744, 2003.
Clemmens, J., H. Hess, R. Lipscomb, Y. Hanein, K. F. Bohringer, C. M. Matzke, G. D. Bachand, B. C. Bunker, and V. Vogel. Mechanisms of microtubule guiding on microfabricated kinesin-coated surfaces: chemical and topographic surface patterns. Langmuir 19:10967–10974, 2003.
Coy, D. L., M. Wagenbach, and J. Howard. Kinesin takes one 8-nm step for each ATP that it hydrolyzes. J. Biol. Chem. 274:3667–3671, 1999.
Dinu, C. Z., J. Opitz, W. Pompe, J. Howard, M. Mertig, and S. Diez. Parallel manipulation of bifunctional DNA molecules on structured surfaces using kinesin-driven microtubules. Small 2:1090–1098, 2006.
Gittes, F., B. Mickey, J. Nettleton, and J. Howard. Flexural rigidity of microtubules and actin-filaments measured from thermal fluctuations in shape. J. Cell. Biol. 120:923–934, 1993.
Guo, Y. X., Y. F. Liu, J. X. Tang, and J. M. Valles. Polymerization force driven buckling of microtubule bundles determines the wavelength of patterns formed in tubulin solutions. Phys. Rev. Lett. 98:198103, 2007.
Hess, H. Self-assembly driven by molecular motors. Soft Matter 2:669–677, 2006.
Hess, H., J. Clemmens, C. Brunner, R. Doot, S. Luna, K. H. Ernst, and V. Vogel. Molecular self-assembly of “nanowires” and “nanospools” using active transport. Nano Lett. 5:629–633, 2005.
Hess, H., J. Clemmens, C. Matzke, G. Bachand, B. Bunker, and V. Vogel. Ratchet patterns sort molecular shuttles. Appl. Phys. A 75:309–313, 2002.
Hess, H., C. M. Matzke, R. Doot, J. Clemmens, G. D. Bachand, B. C. Bunker, and V. Vogel. Molecular shuttles operating undercover: a new photolithographic approach for the fabrication of structured surfaces supporting directed motility. Nano Lett. 3:1651–1655, 2003.
Hiratsuka, Y., T. Tada, K. Oiwa, T. Kanayama, and T. Q. P. Uyeda. Controlling the direction of kinesin-driven microtubule movements along microlithographic tracks. Biophys. J. 81:1555–1561, 2001.
Hutchins, B. M., M. Platt, W. O. Hancock, and M. E. Williams. Motility of CoFe2O4 nanoparticle-labelled microtubules in magnetic fields. Micro Nano Lett. 1:47–52, 2006.
Hutchins, B. M., M. Platt, W. O. Hancock, and M. E. Williams. Directing transport of CoFe2O4-functionalized microtubules with magnetic fields. Small 3:126–131, 2007.
Jia, L. L., S. G. Moorjani, T. N. Jackson, and W. O. Hancock. Microscale transport and sorting by kinesin molecular motors. Biomed. Microdevices 6:67–74, 2004.
Kawamura, R., A. Kakugo, Y. Osada, and J. P. Gong. Microtubule bundle formation driven by ATP: the effect of concentrations of kinesin, streptavidin and microtubules. Nanotechnology 21:145603–145614, 2010.
Kawamura, R., A. Kakugo, K. Shikinaka, Y. Osada, and J. P. Gong. Ring-shaped assembly of microtubules shows preferential counterclockwise motion. Biomacromolecules 9:2277–2282, 2008.
Kawamura, R., A. Kakugo, K. Shikinaka, Y. Osada, and J. P. Gong. Formation of motile assembly of microtubules driven by kinesins. Smart Mater. Struct. 20:129901, 2011.
Kim, T., L. J. Cheng, M. T. Kao, E. F. Hasselbrink, L. J. Guo, and E. Meyhofer. Biomolecular motor-driven molecular sorter. Lab. Chip 9:1282–1285, 2009.
Liu, H., and G. D. Bachand. Understanding energy dissipation and thermodynamics in biomotor-driven nanocomposite assemblies. Soft Matter 7:3087–3091, 2011.
Liu, H. Q., E. D. Spoerke, M. Bachand, S. J. Koch, B. C. Bunker, and G. D. Bachand. Biomolecular motor-powered self-assembly of dissipative nanocomposite rings. Adv. Mater. 20:4476–4481, 2008.
Love, J. C., L. A. Estroff, J. K. Kriebel, R. G. Nuzzo, and G. M. Whitesides. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem. Rev. 105:1103–1169, 2005.
Luria, I., J. Crenshaw, M. Downs, A. Agarwal, S. B. Seshadri, J. Gonzales, O. Idan, J. Kamcev, P. Katira, S. Pandey, T. Nitta, S. R. Phillpot, and H. Hess. Microtubule nanospool formation by active self-assembly is not initiated by thermal activation. Soft Matter 7:3108–3115, 2011.
Martin, B. D., C. M. Soto, A. S. Blum, K. E. Sapsford, J. L. Whitley, J. E. Johnson, A. Chatterji, and B. R. Ratna. An engineered virus as a bright fluorescent tag and scaffold for cargo proteins—capture and transport by gliding microtubules. J. Nanosci. Nanotechnol. 6:2451–2460, 2006.
Moorjani, S. G., L. Jia, T. N. Jackson, and W. O. Hancock. Lithographically patterned channels spatially segregate kinesin motor activity and effectively guide microtubule movements. Nano Lett. 3:633–637, 2003.
Nitta, T., A. Tanahashi, Y. Obara, M. Hirano, M. Razumova, M. Regnier, and H. Hess. Comparing guiding track requirements for myosin- and kinesin-powered molecular shuttles. Nano Lett. 8:2305–2309, 2008.
Pampaloni, F., G. Lattanzi, A. Jonas, T. Surrey, E. Frey, and E. L. Florin. Thermal fluctuations of grafted microtubules provide evidence of a length-dependent persistence length. Proc. Natl. Acad. Sci. USA 103:10248–10253, 2006.
Papra, A., N. Gadegaard, and N. B. Larsen. Characterization of ultrathin poly(ethylene glycol) monolayers on silicon substrates. Langmuir 17:1457–1460, 2001.
Pinot, M., F. Chesnel, J. Z. Kubiak, I. Arnal, F. J. Nedelec, and Z. Gueroui. Effects of confinement on the self-organization of microtubules and motors. Curr. Biol. 19:954–960, 2009.
Ramanathan, M., S. M. Kilbey, Q. M. Ji, J. P. Hill, and K. Ariga. Materials self-assembly and fabrication in confined spaces. J. Mater. Chem. 22:10389–10405, 2012.
Safinya, C. R., D. J. Needleman, M. A. Ojeda-Lopez, U. Raviv, H. P. Miller, and L. Wilson. Higher-order assembly of microtubules by counterions: from hexagonal bundles to living necklaces. Proc. Natl. Acad. Sci. USA 101:16099–16103, 2004.
Service, R. F. How far can we push chemical self-assembly. Science 309:95, 2005.
Stebbings, H., and C. Hunt. The nature of the clear zone around microtubules. Cell Tissue Res. 227:609–617, 1982.
van den Heuvel, M. G. L., C. T. Butcher, R. M. M. Smeets, S. Diez, and C. Dekker. High rectifying efficiencies of microtubule motility on kinesin-coated gold nanostructures. Nano Lett. 5:1117–1122, 2005.
van den Heuvel, M. G. L., M. P. De Graaff, and C. Dekker. Molecular sorting by electrical steering of microtubules in kinesin-coated channels. Science 312:910–914, 2006.
Whitesides, G. M., and B. Grzybowski. Self-assembly at all scales. Science 295:2418–2421, 2002.
Whitesides, G. M., J. P. Mathias, and C. T. Seto. Molecular self-assembly and nanochemistry—a chemical strategy for the synthesis of nanostructures. Science 254:1312–1319, 1991.
Acknowledgments
We thank Nathan Bouxsein and Marlene Bachand for their insight comments, suggestions, and feedback. We also thank Dr. Andrew Boal for intellectual input regarding gold-thiol functionalization to localize kinesin adsorption. This research was supported by the U.S. Department of Energy, Office of Basic Energy Sciences, Division of Materials Sciences and Engineering, Project KC0203010. Sandia National Laboratories is a multi-program laboratory operated by Sandia Corporation, a wholly owned subsidiary of Lockheed Martin Company, for the U.S. Department of Energy’s National Nuclear Security Administration under contract DE-AC04-94AL85000.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Jung-Chi Liao & Henry Hess oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Liu, H., Bachand, G.D. Effects of Confinement on Molecular Motor-Driven Self-Assembly of Ring Structures. Cel. Mol. Bioeng. 6, 98–108 (2013). https://doi.org/10.1007/s12195-012-0256-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-012-0256-5