Abstract
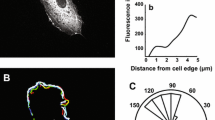
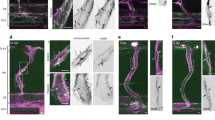
Vascular endothelial cells (ECs) exposed to fluid shear stress (FSS) become elongated and aligned to the direction of flow. However, the process of morphological change in individual cells is different depending of their initial shape. Rac1 and RhoA, members of the family of Rho GTPases, play important roles in cellular morphological changes but are thought to be activated differently in the process. Here, we measured changes in Rac1 and RhoA activities with a focus on the effect of cell orientation when exposed to FSS. In ECs initially oriented parallel to the direction of flow, RhoA and Rac1 were activated primarily in the upstream and the downstream regions of cells, respectively, accompanied by the formation of lamellipodia in the direction of flow. On the other hand, in cells oriented perpendicular to the direction of flow, FSS caused RhoA activation in the upstream region but did not change Rac1 activity. Furthermore, treatment with cytochalasin D inhibited the localization of Rac1 activation and suppressed RhoA activation by FSS. These results indicate that cell orientation affects the local activation of Rac1 and RhoA when induced by forces transmitted through the actin cytoskeleton under a FSS condition.









Similar content being viewed by others
References
Butler, P. J., T. C. Tsou, J. Y. Li, S. Usami, and S. Chien. Rate sensitivity of shear-induced changes in the lateral diffusion of endothelial cell membrane lipids: a role for membrane perturbation in shear-induced MAPK activation. FASEB J. 16:216–218, 2002.
Davies, P. F. Flow-mediated endothelial mechanotransduction. Physiol. Rev. 75:519–560, 1995.
Garnacho, C., V. Shuvaeu, A. Thomas, L. McKenna, J. Sun, M. Koval, S. Albelda, V. Muzylantov, and S. Muro. RhoA activation and actin reorganization involved in endothelial CAM-mediated endocytosis of anti-PECAM carriers: critical role for tyrosine 686 in the cytoplasmic tail of PECAM-1. Blood 111:3024–3033, 2008.
Haidekker, M. A., N. L’Heureux, and J. A. Frangos. Fluid shear stress increases membrane fluidity in endothelial cells: a study with DCVJ fluorescence. Am. J. Physiol. Heart Circ. Physiol. 278:H1401–H1406, 2000.
Itoh, R. E., K. Kurokawa, Y. Ohba, H. Yoshizaki, N. Mochizuki, and M. Matsuda. Activation of Rac1 and Cdc42 video imaged by fluorescent resonance energy transfer-based single molecule probes in the membrane of living cells. Mol. Cell. Biol. 22:6582–6591, 2002.
Kataoka, N., S. Ujita, and M. Sato. The morphological responses of cultured bovine aortic endothelial cells to fluid-imposed shear stress sparse and colony condition. JSME Int. J. Ser. C 41:76–82, 1998.
Nakamura, T., K. Aoki, and M. Matsuda. FRET imaging in nerve growth cones reveals a high level of RhoA activity within the peripheral domain. Mol. Brain Res. 139:277–287, 2005.
Osawa, M., M. Masuda, K. Kusano, and K. Fujiwara. Evidence for a role of platelet endothelial cell adhesion molecule-1 in endothelial cell mechanosignal transduction: is it a mechanoresponsive molecule? J. Cell Biol. 158:773–785, 2002.
Sakamoto, N., T. Ohashi, and M. Sato. Effect of magnetic field on nitric oxide synthesis of cultured endothelial cells. Int. J. Appl. Electromagn. Mech. 14:317–322, 2001.
Sakamoto, N., N. Saito, X. Han, T. Ohashi, and M. Sato. Effect of spatial gradient in fluid shear stress on morphological changes in endothelial cells in response to flow. Biochem. Biophys. Res. Commun. 395:264–269, 2010.
Senapati, S., S. Rachagani, K. Chaudhary, S. L. Johansson, R. K. Singh, and S. K. Batra. Overexpression of macrophage inhibitory cytokine-1 induces metastasis of human prostate cancer cells through the FAK-RhoA signaling pathway. Oncogene 9:1293–1302, 2010.
Shikata, Y., A. Rios, K. Kawkitinarong, N. DePaola, J. G. N. Garcia, and K. G. Birukov. Differential effects of shear stress and cyclic stretch on focal adhesion remodeling, site-specific FAK phosphorylation, and small GTPases in human lung endothelial cells. Exp. Cell Res. 304:40–49, 2005.
Shiu, Y. T., S. Li, W. A. Marganski, S. Usami, M. A. Schwartz, Y. L. Wang, M. Dembo, and S. Chien. Rho mediates the shear-enhancement of endothelial cell migration and traction force generation. Biophys. J. 86:2558–2565, 2004.
Sugaya, Y., N. Sakamoto, T. Ohashi, and M. Sato. Elongation and random orientation of bovine endothelial cells in response to hydrostatic pressure: comparison with response to shear stress. JSME Int. J. Ser. C 46:1248–1255, 2003.
Tzima, E., M. A. D. Pozo, W. B. Kiosses, S. A. Mohamed, S. Li, S. Chien, and M. A. Schwartz. Activation of Rac1 by shear stress in endothelial cells mediates both cytoskeletal reorganization and effects on gene expression. EMBO J. 21:6791–6800, 2002.
Ueki, Y., N. Sakamoto, and M. Sato. Direct measurement of shear strain in adherent vascular endothelial cells exposed to fluid shear stress. Biochem. Biophys. Res. Commun. 394:94–99, 2010.
Ueki, Y., Y. Uda, N. Sakamoto, and M. Sato. Measurements of strain of single stress fibers in living endothelial cells induced by fluid shear stress. Biochem. Biophys. Res. Commun. 395:441–446, 2010.
Vial, E., E. Sahai, and C. J. Marshall. ERK-MAPK signaling coordinately regulates activity of Rac1 and RhoA for tumor cell motility. Cancer Cell 4:67–79, 2003.
Wojcial-Stothard, B., and A. J. Ridley. Shear stress-induced endothelial cell polarization is mediated by Rho and Rac but not Cdc42 or PI 3-kinases. J. Cell Biol. 161:429–439, 2003.
Yoshizaki, H., Y. Ohba, K. Kurokawa, R. E. Itoh, T. Nakashima, N. Mochizuki, K. Nagashima, and M. Matsuda. Activity of Rho-family GTPases during cell division as visualized with FRET-probes. J. Cell Biol. 162:223–232, 2003.
Zaidel-Bar, R., Z. Kam, and B. Geiger. Polarized downregulation of the paxillin-p130CAS-Rac1 pathway induced by shear flow. J. Cell Sci. 118:3997–4007, 2005.
Zondag, G. C. M., E. E. Evers, J. P. Klooster, L. Janssen, R. A. Kammen, and J. G. Collard. Oncogenic Ras downregulates Rac activity, which leads to increased Rho activity and epithelial-mesenchymal transition. J. Cell Biol. 149:775–781, 2000.
Acknowledgments
The authors thank Drs. Ikuo Takahashi and Makoto Takahashi for kindly providing human umbilical cords with the informed consent of donors, and Dr. Kazumasa Ohashi for kindly providing the plasmids. This study was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan (Nos. 20001007 and 21-3835), and The Mitsubishi Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editors Yingxiao Wang & Peter J. Butler oversaw the review of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (MOV 2117 kb)
Supplementary material 2 (MOV 2117 kb)
Supplementary material 3 (MOV 2115 kb)
Supplementary material 4 (MOV 2115 kb)
Rights and permissions
About this article
Cite this article
Nishio, K., Ueki, Y., Sakamoto, N. et al. Effect of Initial Orientation of Vascular Endothelial Cells on Activation of RhoGTPases Induced by Fluid Shear Stress. Cel. Mol. Bioeng. 4, 160–168 (2011). https://doi.org/10.1007/s12195-011-0173-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-011-0173-z