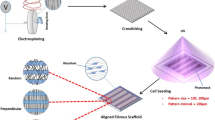
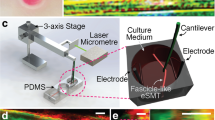
Abstract
Tissue engineering may provide an alternative to cell injection as a therapeutic solution for myocardial infarction. A tissue-engineered muscle patch may offer better host integration and higher functional performance. This study examined the differentiation of skeletal myoblasts on aligned electrospun polyurethane (PU) fibers and in the presence of electromechanical stimulation. Skeletal myoblasts cultured on aligned PU fibers showed more pronounced elongation, better alignment, higher level of transient receptor potential cation channel-1 (TRPC-1) expression, upregulation of contractile proteins and higher percentage of striated myotubes compared to those cultured on random PU fibers and film. The resulting tissue constructs generated tetanus forces of 1.1 mN with a 10-ms time to tetanus. Additional mechanical, electrical, or synchronized electromechanical stimuli applied to myoblasts cultured on PU fibers increased the percentage of striated myotubes from 70 to 85% under optimal stimulation conditions, which was accompanied by an upregulation of contractile proteins such as α-actinin and myosin heavy chain. In describing how electromechanical cues can be combined with topographical cue, this study helped move towards the goal of generating a biomimetic microenvironment for engineering of functional skeletal muscle.









Similar content being viewed by others
References
Alisi A., A. Spaziani, S. Anticoli, M. Ghidinelli, C. Balsano PKR is a novel functional direct player that coordinates skeletal muscle differentiation via p38MAPK/AKT pathways. Cell. Signal. 20: 534–542, 2008
Clark C. B., N. L. McKnight, J. A. Frangos Stretch activation of GTP-binding proteins in C2C12 myoblasts. Exp. Cell Res. 292: 265–273, 2004
Cronin E. M., F. A. Thurmond, R. Bassel-Duby, R. S. Williams, W. E. Wright, K. D. Nelson, H. R. Garner Protein-coated poly(l-lactic acid) fibers provide a substrate for differentiation of human skeletal muscle cells. J. Biomed. Mater. Res. 69A: 373–381, 2004
Engler A. J., M. A. Griffin, S. Sen, C. G. Bonnemann, H. L. Sweeney, D. E. Discher Myotube differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J. Cell Biol. 166: 877–887, 2004
Fernandes S., J. C. Amirault, G. Lande, J. Nguyen, V. Forest, O. Bignolais, G. Lamirault, D. Heudes, J. Orsonneau, M. Heymann, F. Charpentier, P. Lemarchand Autologous myoblast transplantation after myocardial infarction increases the inducibility of ventricular arrhythmias. Cardiovasc. Res. 69: 348–358, 2006
Formigli L., E. Meacci, C. Sassoli, R. Squecco, D. Nosi, F. Chellini, F. Naro, F. Fancini, S. Zecchi-Orlandini Cytoskeleton/stretch-activated ion channel interaction regulates myogenic differentiation of skeletal myoblasts. J. Cell Physiol. 211: 296–306, 2007
Fouts K., B. Fernandes, N. Mal, J. Liu, K. R. Laurita Electrophysiological consequence of skeletal myoblast transplantation in normal and infarcted canine myocardium. Heart Rhythm 3: 452–461, 2006
Fujita H., T. Nedachi, M. Kanzaki Accelerated de novo sarcomere assembly by electric pulse stimulation in C2C12 myotubes. Exp. Cell Res. 313: 1853–1865, 2007
Huang N. F., S. Patel, R. Thakar, J. Wu, B. S. Hsiao, B. Chu, R. J. Lee, S. Li Myotube assembly on nanofibrous and micropatterned polymers. Nano Lett. 6: 537–542, 2006
Huang Y. C., R. G. Dennis, K. Baar Cultured slow vs. fast skeletal muscle cells differ in physiology and responsiveness to stimulation. Am. J. Physiol.-Cell Physiol. 291: 11–17, 2006
Huang Y. C., R. G. Dennis, L. Larkin, K. Baar Rapid formation of functional muscle in vitro using fibrin gels. J. Appl. Physiol. 98: 706–713, 2004
Huber A., A. Pickett, K. M. Shakesheff Reconstruction of spatially orientated myotubes in vitro using electrospun, parallel microfibre arrays. Eur. Cells Mater. 14: 56–63, 2007
Kondoh H., Y. Sawa, S. Miyagawa, S. Sakakida-Kitagawa, I. A. Memon, N. Kawaguchi, N. Mtsuura, T. Shimizu, T. Okano, H. Matsuda Longer preservation of cardiac performance by sheet-shaped myoblast implantation in dilated cardiomyopathic hamsters. Cardiovasc. Res. 69: 466–475, 2006
Kumar A., R. Murphy, P. Robinson, L. Wei, A. M. Boriek Cyclic mechanical strain inhibits skeletal myogenesis through activation of focal adhesion kinase, Rac-1 GTPase, and NF-kappaB transcription factor. FASEB J. 13: 1524–1535, 2004
Lam M. T., S. Sim, X. Zhu, S. Takayama The effect of continuous wavy micropatterns on silicone substrates on the alignment of skeletal muscle myoblasts and myotubes. Biomaterials 27: 4340–4347, 2006
Neumann T., S. D. Hauschka, J. E. Sanders Tissue engineering of skeletal muscle using polymer fiber arrays. Tissue Eng. 9: 995–1003, 2003
Omoteyama K., Y. Mikami, M. Takagi Foxc2 induces expression of MyoD and differentiation of the mouse myoblast cell line C2C12. Biochem. Biophys. Res. Commun. 358: 885–889, 2007
Otis J. S., T. J. Burkholder, G. K. Pavlath Stretch-induced myoblast proliferation is dependent on the COX2 pathway. Exp. Cell Res. 2: 417–425, 2005
Pedrotty D. M., J. Koh, B. H. Davis, D. A. Taylor, P. Wolf, L. E. Niklason Engineering skeletal myoblasts: roles of three-dimensional culture and electrical stimulation. Am. J. Physiol.-Heart Circ. Physiol. 288: 1620–1626, 2005
Powell C. A., B. L. Smiley, J. Mills, H. H. Vandenburgh Mechanical stimulation improves tissue-engineered human skeletal muscle. Am. J. Physiol.-Cell Physiol. 5: 1557–1565, 2002
Ramos G. A., J. M. Hare Cardiac cell-based therapy: cell types and mechanisms of actions. Cell Transplant. 16: 951–961, 2007
Rhim C., D. A. Lowell, M. C. Reedy, D. H. Slentz, S. J. Zhang, W. E. Kraus, G. A. Truskey Morphology and ultrastructure of differentiating three-dimensional mammalian skeletal muscle in a collagen gel. Muscle Nerve 36: 71–80, 2007
Sakiyama K., S. Abe, Y. Tamatsu, Y. Ide Effects of stretching stress on the muscle contraction proteins of skeletal muscle myoblasts. Biomed. Res. 26: 61–68, 2005
Sbrana F., C. Sassoli, E. Meacci, D. Nosi, R. Squecco, F. Paternostro, B. Tiribilli, S. Zecchi-Orlandini, F. Francin, L. Formigli Role of stress fiber contraction in surface tension development and stretch-activated channel regulation in C2C12 myoblasts. Am. J. Physiol.-Cell Physiol. 295: 160–172, 2008
Siepe M., M. N. Giraud, E. Liljensten, U. Nydegger, P. Menasche, T. Carrel, H. T. Tevaearai, Construction of skeletal myoblast-based polyurethane scaffolds for myocardial repair. Artif. Organs 6: 425–433, 2007
Tatsumi R., S. M. Sheehan, H. Iwasaki, A. Hattori, R. E. Allen Mechanical stretch induces activation of skeletal muscle satellite cells in vitro. Exp. Cell Res. 1: 107–114, 2001
Thelen M. H., W. S. Simonides, C. van Hardeveld Electrical stimulation of C2C12 myotubes induces contractions and represses thyroid-hormone-dependent transcription of the fast-type sacroplasmic-reticulum Ca2+-ATPase gene. Biochem. J. 321: 845–848, 1997
Woo J. S., D. H. Kim, P. D. Allen, E. H. Lee TRPC3-interacting triadic proteins in skeletal muscle. Biochem. J. 411: 399–405, 2008
Yamamoto, D. L., R. I. Csikasz, Y. Li, G. Sharma, K. Hjort, R. Karlsson, and T. Bengtsson. Myotube formation on micro-patterned glass: intracellular organization and protein distribution in C2C12 skeletal muscle cells. J. Histochem. Cytochem. 10:881–892, 2008.
Yan W., S. George, U. Fotadar, N. Tyhovych, A. Kamer, M. J. Yost, R. L. Price, C. R. Haggart, J. W. Holmes, L. Terracio Tissue engineering of skeletal muscle. Tissue Eng. 13: 2781–2790, 2007
Yim E. K., S. W. Pang, K. W. Leong Synthetic nanostructures inducing differentiation of human mesenchymal stem cells into neuronal lineage. Exp. Cell Res. 9: 1820–1829, 2007
Yim E. K., R. M. Reano, S. W. Pang, A. F. Yee, C. S. Chen, K. W. Leong Nanopattern-induced changes in morphology and motility of smooth muscle cells. Biomaterials 26: 5405–5413, 2005
Zhang S. J., G. A. Truskey, W. E. Kraus Effect of cyclic stretch on beta1D-integrin expression and activation of FAK and RhoA. Am. J. Physiol.-Cell Physiol. 6: 57–69, 2007
Acknowledgments
The authors would like to acknowledge the contribution of Dr. George Truskey, Caroline Rhim and Matt Brown. Support by NIH Grants EB003447 to Kam W. Leong and AR055226 to Nenad Bursac are also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liao, IC., Liu, J.B., Bursac, N. et al. Effect of Electromechanical Stimulation on the Maturation of Myotubes on Aligned Electrospun Fibers. Cel. Mol. Bioeng. 1, 133–145 (2008). https://doi.org/10.1007/s12195-008-0021-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-008-0021-y