Abstract
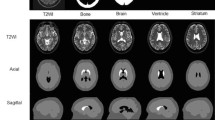
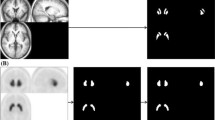
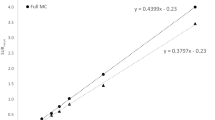
This study aimed to evaluate the effect of ventricular enlargement on the specific binding ratio (SBR) and to validate the cerebrospinal fluid (CSF)-Mask algorithm for quantitative SBR assessment of 123I-FP-CIT single-photon emission computed tomography (SPECT) images with the use of a 3D-striatum digital brain (SDB) phantom. Ventricular enlargement was simulated by three-dimensional extensions in a 3D-SDB phantom comprising segments representing the striatum, ventricle, brain parenchyma, and skull bone. The Evans Index (EI) was measured in 3D-SDB phantom images of an enlarged ventricle. Projection data sets were generated from the 3D-SDB phantoms with blurring, scatter, and attenuation. Images were reconstructed using the ordered subset expectation maximization (OSEM) algorithm and corrected for attenuation, scatter, and resolution recovery. We bundled DaTView (Southampton method) with the CSF-Mask processing software for SBR. We assessed SBR with the use of various coefficients (f factor) of the CSF-Mask. Specific binding ratios of 1, 2, 3, 4, and 5 corresponded to SDB phantom simulations with true values. Measured SBRs > 50% that were underestimated with EI increased compared with the true SBR and this trend was outstanding at low SBR. The CSF-Mask improved 20% underestimates and brought the measured SBR closer to the true values at an f factor of 1.0 despite an increase in EI. We connected the linear regression function (y = − 3.53x + 1.95; r = 0.95) with the EI and f factor using root-mean-square error. Processing with CSF-Mask generates accurate quantitative SBR from dopamine transporter SPECT images of patients with ventricular enlargement.






Similar content being viewed by others
References
Günther I, Hall H, Halldin C, Swahn CG, Farde L, Sedvall G. [125I] beta-CIT-FE and [125I] beta-CIT-FP are superior to [125I] beta-CIT for dopamine transporter visualization: auto-radiographic evaluation in human brain. Nucl Med Biol. 1997;24:629–34.
Piggot M, Perry E, Marshall EF, McKeith IG, Johnson M, Melrose HL, Court JA, Lloyd S, Fairbairn A, Brown A, Thompson P, Perry RH. Nigrostriatal dopaminergic activities in dementia with Lewy bodies in relation to neuroleptic sensitivity: comparisons with Parkinson’s disease. Soc Bio Psychiatry. 1998;44:765–74.
Booij J, Tissingh G, Boer GJ, Speelman JD, Stoof JC, Janssen AG, Wolters EC, van Roven EA. [123I] FP-CIT SPECT shows a pronounced decline of striatal dopamine transporter labelling in early and advanced Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1997;62:133–40.
Booij J, Speelman JD, Horstink MW, Wolters EC. The clinical benefit of imaging striatal dopamine transporters with [123I] FP-CIT SPECT in differentiating patients with presynaptic Parkinsonism from those with other forms of Parkinsonism. Eur J Nucl Med. 2001;28:266–72.
Antonini A, Benti R, De Notaris R, Tesei S, Zecchinelli A, Sacilotto G, Meucci N, Canesi M, Mariani C, Pezzoli G, Gerundini P. 123I-loflupane/SPECT binding to striatal dopamine transporter (DAT) uptake in patients with Parkinson’s disease, multiple system atrophy, and progressive supranuclear palsy. Neurol Sci. 2003;24:149–50.
Tossici-Bolt L, Hoffmann SM, Kemp PM, Mehta RL, Fleming JS. Quantification of [123I] FP-CIT SPECT brain images: an accurate technique for measurement of the specific binding ratio. Eur J Nucl Med Mol Imaging. 2006;33:1491–9.
Fleming JS, Bolt L, Stratford JS, Kemp PM. The specific uptake size index for quantifying radiopharmaceutical uptake. Phys Med Biol. 2004;49:227–34.
Calvini P, Rodriquez G, Inguglia F, Mignone A, Guerra UP, Nobili F. The basal ganglia matching tools package for striatal uptake semi-quantification: description and validation. Eur J Nucl Med Mol Imaging. 2007;34:1240–53.
Matsuda H, Mizumura S, Soma T, Takemura N. Conversion of brain SPECT images between different collimators and reconstruction processes for analysis using statistical parametric mapping. Nucl Med Commun. 2004;25:67–74.
Evans WA Jr. An encephalographic ratio for estimating the size of the cerebral ventricles. Arch Neurol Psychiatry. 1942;47(6):931–37.
Ishii K, Kawaguchi T, Shimada K, Ohkawa S, Miyamoto N, Kanda T. Uemura T, Yoshikawa T, Mori E. Voxel-based analysis of gray matter and CSF space in idiopathic normal pressure hydrocephalus. Dement Geriatr Cognit Disord. 2008;25:329–35.
Nonokuma M, Kuwabara Y, Hida K, Tano T, Takano K, Yoshimitsu K. Optimal ROI setting on the anatomically normalized I-123 FP-CIT images using high-resolution SPECT. Ann Nucl Med. 2016;30:637–44.
Furuta A, Onishi H. Realistic 3D-striatum digital brain (3D-SDB) phantom studied for its effect on ventricle in the brain for semiquantitative index of specific binding ratio. Nihon Hoshasen Gijutsu Gakkai Zasshi. 2017;73:1018–27 (Japanese).
Nishikawa K. Image processing apparatus, image processing method, and program Japan Patent: WO 2016152448 A1. 2016.09.29 http://www.google.com/patents/WO2016152448A1?cl=en.
Maeda H, Yamaki N, Azuma M. Development of the software package of the nuclear medicine data processor for education and research. Nihon Hoshasen Gijutsu Gakkai Zasshi. 2012;68:299–306 (Japanese).
Yokoi T, Shinohara H, Onishi H. Performance evaluation of OSEM reconstruction algorithm incorporating three-dimensional distance-dependent resolution compensation for brain SPECT: a simulation study. Ann Nucl Med. 2002;16:11–8.
Yamaki N, Natsume T, Takeda K, Maeda H, Hasebe S, Kinda A, Motomura N. Simultaneous spatial resolution correction in SPECT reconstruction using OS-EM algorithm. Jpn J Med Phys. 2004;24:61–71 (Japanese).
Onishi h, Motomura N, Fujino K, Natsume T, Haramoto Y. Quantitative performance of advanced resolution recovery strategies on SPECT images: evaluation with use of digital phantom models. Radiol Phys Technol. 2013;6:42–53.
Ng SE, Low AM, Tang KK, Chan YH, Kwok RK. Value of quantitative MRI biomarkers (Evans’ index, aqueductal flow rate, and apparent diffusion coefficient) in idiopathic normal pressure hydrocephalus. J Magn Reson Imaging. 2009;30(4):708–15.
Kanda Y. Investigation of the freely available easy-to-use software EZR for medical statistics. Bone Marrow Transpl. 2013;48:452–58.
Toma AK, Holl E, Kitchen ND. Watkins LD. Evans’ index revisited: the need for an alternative in normal pressure hydrocephalus. Neurosurgery. 2011;68(4):939–44.
Mori E, Ishikawa M, Kato T, Kazui H, Miyake H, Miyajima M. Nakajima M, Hashimoto M, Kuriyama N, Tokuda T, Ishii K, Kaijima M, Hirata Y, Saito M, Arai H. Guidelines for management of idiopathic normal pressure hydrocephalus: second edition. Neuro Med Chir. 2012;52:775–809.
Crespo C, Gallego J, Cot A, Falcon C, Bullich S, Pareto D. Aquiar P, Sempau J, Lomena F, Calvino F, Pavia J, Ros D. Quantification of dopaminergic neurotransmission SPECT studies with 123I-labelled radioligands. A comparison between different imaging systems and data acquisition protocols using Monte Carlo simulation. Eur J Nucl Med Mol Imaging. 2008;35:1334–42.
Acknowledgements
This study was supported by the Digital Image Scientific Research Meeting (Mihara, Hiroshima, Japan).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Statements of human rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Statements of animal rights
This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
All other authors declare that they have no conflicts of interest.
About this article
Cite this article
Furuta, A., Onishi, H. & Amijima, H. Quantitation of specific binding ratio in 123I-FP-CIT SPECT: accurate processing strategy for cerebral ventricular enlargement with use of 3D-striatal digital brain phantom. Radiol Phys Technol 11, 219–227 (2018). https://doi.org/10.1007/s12194-018-0459-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12194-018-0459-0