Abstract
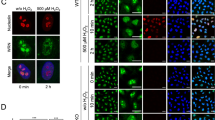
For many years, there has been uncertainty concerning the reason for Hsp70 translocation to the nucleus and nucleolus. Herein, we propose that Hsp70 translocates to the nucleus and nucleoli in order to participate in pathways related to the protection of the nucleoplasmic DNA or ribosomal DNA from single-strand breaks. The absence of Hsp70 in HeLa cells, via Hsp70 gene silencing (knockdown), indicated the essential role of Hsp70 in DNA integrity. Therefore, HeLa Hsp70 depleted cells were very sensitive in heat treatment and their DNA breaks were multiple compared to that of control HeLa cells. The molecular mechanism with which Hsp70 performs its role at the level of nucleus and nucleolus during stress was examined. Hsp70 co-localizes with PARP1 in the nucleus/nucleoli as was observed in confocal studies and binds to the BCRT domain of PARP1 as was revealed with protein–protein interaction assays. It was also found that Hsp70 binds simultaneously to XRCC1 and PARP-1, indicating that Hsp70 function takes place at the level of DNA repair and possibly at the base excision repair system. Making a hypothetical model, we have suggested that Hsp70 is the molecule that binds and interrelates with PARP1 creating the repair proteins simultaneously, such as XRCC1, at the single-strand DNA breaks. Our data partially clarify a previously unrecognized cellular response to heat stress. Finally, we can speculate that Hsp70 plays a role in the quality and integrity of DNA.









Similar content being viewed by others
Abbreviations
- NCA:
-
Nucleolar comet assay
References
Adachi H, Katsuno M, Minamiyama M, Sang C, Pagoulatos G, Angelidis C, Kusakabe M, Yoshiki A, Kobayashi Y, Doyu M, Sobue G (2003) Heat shock protein 70 chaperone overexpression ameliorates phenotypes of the spinal and bulbar muscular atrophy transgenic mouse model by reducing nuclear-localized mutant androgen receptor protein. J Neurosci 23:2203–2211
Andersen JS, Lam YW, Leung AK, Ong SE, Lyon CE, Lamond AI, Mann M (2005) Nucleolar proteome dynamics. Nature 433:77–83 doi:10.1038/nature03207
Angelidis Ch, Lazaridis I, Pagoulatos G (1988) Specific inhibition of simian virus 40 protein synthesis by heat and arsenite treatment. Eur J Biochem 172:27–34 doi:10.1111/j.1432-1033.1988.tb13851.x
Angelidis Ch, Lazaridis I, Pagoulatos G (1991) Constitutive expression of heat-shock protein 70 in mammalian cells confers thermoresistance. Eur J Biochem 199:35–39 doi:10.1111/j.1432-1033.1991.tb16088.x
Angelidis CE, Nova C, Lazaridis I, Kontoyiannis D, Kollias G, Pagoulatos GN (1996) Overexpression of HSP70 in transgenic mice results in increased cell thermotolerance. Transgenics 2:111–117
Angelidis CE, Lazaridis I, Pagoulatos GN (1999) Aggregation of hsp70 and hsc70 in vivo is distinct and temperature-dependent and their chaperone function is directly related to non-aggregated forms. Eur J Biochem 259:505–512 doi:10.1046/j.1432-1327.1999.00078.x
Bases R (2006) Heat shock protein 70 enhanced deoxyribonucleic acid base excision repair in human leukemic cells after ionizing radiation. Cell Stress Chaperones 11:240–249 doi:10.1379/CSC-185R.1
Beckman RP, Mizzen LA, Welch WJ (1990) Interaction of hsp70 with newly synthesized proteins: Implication s for protein folding and assembly. Science 248:850–854 doi:10.1126/science.2188360
Benaroudj N, Triniolles F, Ladjimi MM (1996) Effect of nucleotides, peptides and unfolded proteins on the self-association of the molecular chaperone hsc70. J Biol Chem 271:18471–18476 doi:10.1074/jbc.271.31.18471
Bozidis P, Lazaridis I, Pagoulatos G, Angelidis C (2002) Mydj2 as a potent partner of hsc70 in mammalian cells. Eur J Biochem 269:1553–1560 doi:10.1046/j.1432-1033.2002.02807.x
Bruskov VI, Malakhova LV, Masalimov ZK, Chernikov AV (2002) Heat-induced formation of reactive oxygen species and 8-oxoguanine, a biomarker of damage to DNA. Nucleic Acid Res 30:1354–1363 doi:10.1093/nar/30.6.1354
Bukau B, Weissman J, Horwich A (2006) Molecular chaperones and protein quality control. Cell 125:443–451 doi:10.1016/j.cell.2006.04.014
Caldecott KW (2003) XRCC1 and DNA strand break repair. DNA Repair (Amst) 2:955–969 doi:10.1016/S1568-7864(03)00118-6
Caldecott KW, Aoufouchi S, Johnson P, Shall S (1996) XRCC1 polypeptide interacts with DNA polymerase beta and possibly poly(ADP-ribose) polymerase, and DNA ligase III is a novel molecular “nick sensor” in vitro. Nucleic Acids Res 24:4387–4394 doi:10.1093/nar/24.22.4387
Calini V, Urani C, Camatini M (2003) Overexpression of Hsp70 is induced by ionizing radiation in C3H 10T1/2 cells and protects from DNA damage. Toxicol In Vitro 17:561–566 doi:10.1016/S0887-2333(03)00116-4
Chiang H-L, Terlecky SR, Plant CP, Dice JF (1989) A role for a 70-kilodalton heat shock protein in lysosomal degredation of intracellular proteins. Science 246:382–385 doi:10.1126/science.2799391
Chirico W, Waters MG, Blobel G (1988) 70 K heat shock related proteins stimulate protein translocation into microsomes. Nature 332:805–810 doi:10.1038/332805a0
Collins AR, Ma AG, Duthie SJ (1995) The kinetics of repair of oxidative DNA damage (strand breaks and oxidised pyrimidines) in human cells. Mutat Res 336:69–77
Coute Y, Burgess JA, Diaz J-J, Chichester C, Lisacek F, Greco A, Sanchez J-C (2005) Deciphering the human nucleolar proteome. Mass Spectrom Rev 25:215–234 doi:10.1002/mas.20067
Cummings CJ, Sun Y, Opal P, Antalffy B, Mestril R, Orr HT, Dillmann WH, Zoghbi HY (2001) Over-expression of inducible HSP70 chaperone suppresses neuropathology and improves motor function in SCA1 mice. Hum Mol Genet 10:1511–1518 doi:10.1093/hmg/10.14.1511
Dantzer F, Giraud-Panis M-J, Jaco I, Amé J-C, Schultz I, Blasco M, Koering C-El, Gilson E, de Murcia JM, de Murcia G, Schreiber V (2004) Functional Interaction between poly(ADP-Ribose) polymerase 2 (PARP-2) and TRF2: PARP activity negatively regulates TRF2. Mol Cell Biol 24:1595–1607 doi:10.1128/MCB.24.4.1595-1607.2004
Doulias P-T, Christoforidis S, Brunk UT, Galaris D (2003) Endosomal and lysosomal effects of desferrioxamine: protection of HeLa cells from hydrogen peroxide-induced DNA damage and induction of cell-cycle arrest. Free Radic Biol Med 35:719–728 doi:10.1016/S0891-5849(03)00396-4
Doulias P-T, Kotoglou P, Tenopoulou M, Keramisanou D, Tzavaras T, Brunk U, Galaris D, Angelidis C (2007) Involvement of heat shock protein-70 in the mechanism of hydrogen peroxide-induced DNA damage: the role of lysosomes and iron. Free Radic Biol Med 42:567–577 doi:10.1016/j.freeradbiomed.2006.11.022
Dressel R, Johnson JP, Gunther E (1998) Heterogeneous patterns of constitutive and heat shock induced expression of HLA-linked HSP70-1 and HSP70-2 heat shock genes in human melanoma cell lines. Melanoma Res 8:482–492 doi:10.1097/00008390-199812000-00002
Flaherty KM, DeLuca-Flaherty C, McKay DB (1990) Three dimensional structure of the ATPase fragment of a 70 K heat-shock cognate protein. Nature 346:623–628 doi:10.1038/346623a0
Gilbert CS, Van den Bosch M, Green MC, Vialard JE, Grenon M, Erdjument-Bromage H, Tempst P, Lowndes NF (2003) The budding yeast Rad9 checkpoint complex: chaperone proteins are required for its function. EMBO Rep 4:953–958 doi:10.1038/sj.embor.embor935
Grawunder U, Zimmer D, Lieber RM (1998) DNA ligase IV binds to XRCC4 via a motif located between rather than within its BRCT domains. Curr Biol 8:873–879 doi:10.1016/S0960-9822(07)00349-1
Gwack Y, Nakamura H, Lee SH, Souvlis J, Yustein JT, Gygi S, Kung HJ, Jung JU (2003) Poly(ADP-Ribose)polymerase I and Ste20-like kinase hKFC act as transcriptional repressors for gamma-2 herpesvirus lytic replication. Mol Cell Biol 23:8282–8294 doi:10.1128/MCB.23.22.8282-8294.2003
Hang H, Fox MH (1995) Expression of hsp70 induced in CHO cells by 45°C hyperthermia is cell cycle associated and DNA synthesis dependent. Cytometry 19:119 doi:10.1002/cyto.990190206
Henderson AS, Warburton D, Atwood KC (1972) Location of ribosomal DNA in the human chromosome complement. Proc Natl Acad Sci U S A 69:3394–3398 doi:10.1073/pnas.69.11.3394
Hightower LE (1991) Heat shock, stress proteins, chaperones, and proteotoxicity. Cell 66:191–197 doi:10.1016/0092-8674(91)90611-2
Huang H-C, Sherman My, Kandror O, Goldberg AL (2001) The molecular chaperone DnaJ is required for the degradation of a soluble abnormal protein in E. coli. J Biol Chem 276:3920–3926 doi:10.1074/jbc.M002937200
Huyton T, Bates PA, Zhang X, Sternberg MJE, Freemont PS (2000) The BRCA1 C-terminal domain: structure and function. Mutat Res 460:319–332
Ikejima M, Noguchi S, Yamashita R, Ogura T, Sugimura T, Gill DM, Miwa M (1990) The zinc fingers of human poly(ADP-ribose) polymerase are differentially required for the recognition of DNA breaks and nicks and the consequent enzyme activation. Other structures recognize intact DNA. J Biol Chem 265:21907–21913
Kampinga HH, Hageman J, Vos MJ, Kubota H, Tanguay RM, Bruford EA, Cheetham ME, Chen B, Hightower LE (2009) Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones 14:105–111 doi:10.1007?s12192-008-0068-7
Lindquist S, Craig EA (1988) The heat shock proteins. Annu Rev Genet 22:631–677 doi:10.1146/annurev.ge.22.120188.003215
Makatsori D, Kourmouli N, Polioudaki H, Shultz LD, McLean K, Theodoropoulos PA, Singh PB, Georgatos SD (2004) The inner nuclear membrane protein lamin B receptor forms distinct microdomains and links epigenetically marked chromatin to the nuclear envelope. J Biol Chem 279:25567–25573 doi:10.1074/jbc.M313606200
Masson M, Niedergang C, Schreiber V, Muller S, Menissier-de Murcia J, deMurcia G (1998) XRCC1 is specifically associated with poly(ADP-ribose) polymerase and negatively regulates its activity following DNA damage. Mol Cell Biol 18:3563–3571
Mendez F, Kozin E, Bases R (2003) Heat shock protein 70 stimulation of the deoxyribonucleic acid base excision repair enzyme polymerase beta. Cell Stress Chaperones 8:153–161 doi:10.1379/1466-1268(2003)008<0153:HSPSOT>2.0.CO;2
Milarski KL, Morimoto RI (1986) Expression of human Hsp70 during the synthetic phase of the cell cycle. Proc Natl Acad Sci U S A 83:9517–9521 doi:10.1073/pnas.83.24.9517
Milarski KL, Morimoto RI (1989) Mutational analysis of the human hsp70 protein: distinct domains for nucleolar localization and ATP-binding. J Cell Biol 109:1947–1962 doi:10.1083/jcb.109.5.1947
Minami Y, Hohfeld J, Ohtsuka K, Hartl F-U (1996) Regulation of the heat shock protein 70 reaction cycle by the mammalian DnaJ homolog, hsp40. J Biol Chem 271:19617–19624 doi:10.1074/jbc.271.5.2641
Morimoto RI, Tissières A, Georgopoulos C (1990) The stress response, functions of the proteins, and perspectives. In: Morimoto RI, Tissières A, Georgopoulus C (eds) Stress proteins in biology and medicine. Cold Spring Harbor Laboratory Press, New York, pp 1–36
Mosser DD, Morimoto RI (2004) Molecular chaperones and the stress of oncogenesis. Oncogene 23:2907–2918 doi:10.1038/sj.onc.1207529
Muramatsu M, Onishi T (1978) Isolation and purification of nucleoli and nucleolar chromatin from mammalian cells. Methods Cell Biol 17:141–161 doi:10.1016/S0091-679X(08)61142-5
Nazarkina ZK, Khodyreva SN, Marsin S, Lavrik OI, Radicella JP (2007) XRCC1 interactions with base excision repair DNA intermediates. DNA Repair 6:254–264 doi:10.1016/j.dnarep.2006.10.002
Niu P, Liu L, Gong Z, Tan H, Wang F, Yuan J, Feng Y, Wei Q, Tangway RM, Wu T (2006) Overexpressed heat shock protein 70 protects cells against DNA damage caused by ultraviolet C in a dose-dependent manner. Cell Stress Chaperones 11:162–169 doi:10.1379/CSC-175R.1
Nollen EA, Salomons FA, Brunsting JF, Want JJ, Sibon OC, Kampinga HH (2001) Dynamic changes in the localization of thermally unfolded nuclear proteins associated with chaperone-dependent protection. Proc Natl Acad Sci U S A 98:12038–12043 doi:10.1073/pnas.201112398
Ochs RL (1998) Methods used to study structure and function of the nucleolus. Methods Cell Biol 53:303–321 doi:10.1016/S0091-679X(08)60884-5
Plumier J-CL, Ross BM, Currie RW, Angelidis CH, Kazlaris H, Kollias G, Pagoulatos GN (1995) Transgenic mice expressing the human hsp70 have improved post-ischemic myocardial recovery. J Clin Invest 95:1854–1860 doi:10.1172/JCI117865
Reddy MV, Gangadharam PRJ (1992) Heat shock treatment of macrophages causes increased release of superoxide anion. Infect Immun 60:2386–2390
Rice PA (1999) Holding damaged DNA together. Nat Struct Biol 6:805–806 doi:10.1038/12257
Rodriguez M, Yu X, Chen J, Songyang Z (2003) Phosphopeptide binding specificities of BRCA1 COOH-terminal (BRCT) domains. J Biol Chem 278:52914–52918 doi:10.1074/jbc.C300407200
Sainis I, Angelidis C, Pagoulatos GN, Lazardis I (2000) Hsc70 interactions with SV40 viral proteins differ between per missive and non-permissive mammalian cells. Cell Stress Chaperones 5:132–138 doi:10.1379/1466-1268(2000)005<0132:HIWSVP>2.0.CO;2
Saliba RS, Munro PM, Luthert PJ, Cheetham ME (2002) The cellular fate of mutant rhodopsin: quality control, degradation and aggresome formation. J Cell Sci 115:2907–2918
Salma A, Tsiapos A, Lazaridis I (2007) The viral SV40 T antigen cooperates with dj2 to enhance hsc70 chaperone function. FEBS J 274:5021–5027 doi:10.1111/j.1742-4658.2007.06019.x
Samali A, Cotter TG (1996) Heat shock proteins increase resistance to apoptosis. Exp Cell Res 223:163–170 doi:10.1006/excr.1996.0070
Sambrook J, Fritsch EF, Maniatis TM (1989) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, USA
Schreiber V, Dantzer F, Ame J-C, De Murcia G (2006) Poly(ADP-ribose): novel functions for an old molecule. Nat Rev Mol Cell Biol 7:517–528 doi:10.1038/nrm1963
Scott MD, Frydman J (2003) Aberrant protein folding as the molecular basis of cancer. Methods Mol Biol 232:67–76
Wang TF, Chang JH, Wang C (1993) Identification of the peptide binding domain of hsc70. 18-Kilodalton fragment located immediately after ATPase domain is sufficient for high affinity binding. J Biol Chem 268:26049–26051
Welch WJ, Feramisco JR (1984) Nuclear and nucleolar localization of the 72,000-dalton heat shock protein in heat-shocked mammalian cells. J Biol Chem 259:4501–4513
Zhang X, Morena S, Bates PA, Whitehead C, Coffer AI, Hainbucher K, Nash R, Sternbera JE, Lindahk T, Freemont PS (1998) Structure of an XRCC1 BRCT domain: a new protein–protein interaction module. EMBO J 17:6404–6411 doi:10.1093/emboj/17.21.6404
Acknowledgments
We are very thankful to Professor of Biochemistry, Molecular Biology, and Cell Biology, R. Morimoto., Professor of Biological Chemistry D. Galaris and Emeritus Professor Pagoulatos Gerassimos for their support with reagents or useful discussions. We also thank Dr. Doulias P-T., Dr. Skyrlas A., Dr. Markopoulos G., Dr. Noutsopoulos D., and Dr. Zerikiotis S. for their technical and scientific support. This research was partially co-funded by the European Union and the Hellenic Ministry of Education (program “Herakleitos”) within the “Operational Program for Education and Initial Vocational Training. It was also partially supported by a grant to P. Vezyraki from the Empeirikio Institution, Athens.
Author information
Authors and Affiliations
Corresponding author
Additional information
Outlining prior scientific knowledge on the subject and novel information: The role of Hsp70 translocation to the nucleus and nucleolus during heat stress has been nearly unknown. It has been proposed that this biological phenomenon is correlated to Hsp70-chaperoning activity. Furthermore, some previous observations in yeast have revealed that Rad9 complexes—Rad9 being the prototype DNA-damage checkpoint gene—contain Ssa1 and or Ssa2 chaperone proteins, both reconstituting the functions of the corresponding Hsp70 in mammalian cells. Here, we propose that Hsp70 translocates to the nuclei/nucleoli during heat stress, binds to PARP-1 and/or XRCC1, and protects HeLa cells from increased single-strand DNA breaks.
Rights and permissions
About this article
Cite this article
Kotoglou, P., Kalaitzakis, A., Vezyraki, P. et al. Hsp70 translocates to the nuclei and nucleoli, binds to XRCC1 and PARP-1, and protects HeLa cells from single-strand DNA breaks. Cell Stress and Chaperones 14, 391–406 (2009). https://doi.org/10.1007/s12192-008-0093-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-008-0093-6