Abstract
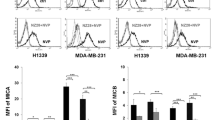
This study compared the effects of the human 70-kDa stress protein (Hsp70) peptide, TKDNNLLGRFELSG (TKD), proinflammatory cytokines, or a combination of both on the repertoire of receptors expressed by human natural killer (NK) cells and their capacity to kill human CX colon carcinoma cells, K562 erythroleukemic cells, and leukemic blasts from two patients with acute myelogenous leukemia. Low-dose interleukin (IL) 2/IL-15 and TKD increase the expression density of activatory (NKG2D, NKp30, NKp44, NKp46, CD94/NKG2C) and inhibitory (CD94/NKG2A) receptors on NK cells. Concomitantly, IL-2/TKD treatment enhances the cytotoxicity of NK cells (as reflected by their secretion of granzyme B) against Hsp70 membrane-positive and human leukocyte antigen (HLA)-E membrane-negative (Hsp70+/HLA-E−) CX+ and K562 cells. However, it had no effect on the responsiveness to Hsp70−/HLA-E− CX− cells over that induced by IL-2 alone. The cytotoxicity of IL-2/TKD-activated, purified NK cells and peripheral blood mononuclear cells against Hsp70+/HLA-E+ leukemic blasts was weaker than that against Hsp70+/HLA-E− K562 cells. Hsp70-blocking and HLA-E transfection experiments confirmed membrane-bound Hsp70 as being a recognition/activatory ligand for NK cells, as cytotoxicity was reduced by the presence of the anti-Hsp70 monoclonal antibody cmHsp70.2 and by inhibiting Hsp70 synthesis using short interference ribonucleic acid. HLA-E was confirmed as an inhibitory ligand, as the extent of NK cell-mediated lysis of K562 cell populations that had been transfected with HLA-ER or HLA-EG alleles was dependent on the proportion of HLA-E-expressing cells. These findings indicate that Hsp70 (as an activatory molecule) and HLA-E (as an inhibitory ligand) expression influence the susceptibility of leukemic cells to the cytolytic activities of cytokine/TKD-activated NK cells.





Similar content being viewed by others
References
Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T (1999) Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 285:727–729
Botzler C, Issels R, Multhoff G (1996) Heat-shock protein 72 cell-surface expression on human lung carcinoma cells in association with an increased sensitivity to lysis mediated by adherent natural killer cells. Cancer Immunol Immunother43:226–230
Botzler C, Li G, Issels RD, Multhoff G (1998) Definition of extracellular localized epitopes of Hsp70 involved in an NK immune response. Cell Stress Chaperones 3:6–11
Cosman D, Mullberg J, Fanslow W, Armitage R, Chin W, Cassiano I (2000) The human cytomegalovirus (HCMV) glycoprotein, UL16, binds to the MHC class I-related protein, MICB/PERB11, and to two novel, MHC class I-related molecules. FASEB J 14:A1018
Farag SS, Caligiuri MA (2006) Human natural killer cell development and biology. Blood Rev 20:123–137
Farag SS, Fehniger T, Ruggeri L, Velardi A, Caligiuri MA (2002) Natural killer cells: biology and application in stem-cell transplantation. Cytotherapy 4:445–446
Fehniger TA, Caligiuri MA (2001) Interleukin 15: biology and relevance to human disease. Blood 97:14–32
Gross C, Hansch D, Gastpar R, Multhoff G (2003a) Interaction of heat shock protein 70 peptide with NK cells involves the NK receptor CD94. Biol Chem 384:267–279
Gross C, Koelch W, DeMaio A, Arispe N, Multhoff G (2003b) Cell surface-bound heat shock protein 70 (Hsp70) mediates perforin-independent apoptosis by specific binding and uptake of granzyme B. J Biol Chem 278:41173–41181
Gross C, Schmidt-Wolf IG, Nagaraj S, Gastpar R, Ellwart J, Kunz-Schughart LA, Multhoff G (2003c) Heat shock protein 70-reactivity is associated with increased cell surface density of CD94/CD56 on primary natural killer cells. Cell Stress Chaperones 8:348–360
Houchins JP, Lanier LL, Niemi EC, Phillips JH, Ryan JC (1997) Natural killer cell cytolytic activity is inhibited by NKG2-A and activated by NKG2-C. J Immunol 158:3603–3609
Kirwan SE, Burshtyn DN (2007) Regulation of natural killer cell activity. Curr Opin Immunol 19:46–54
Lanier LL, Ruitenberg JJ, Phillips JH (1988) Functional and biochemical analysis of CD16 antigen on natural killer cells and granulocytes. J Immunol 141:3478–3485
Lanier LL, Corliss B, Wu J, Phillips JH (1998) Association of DAP12 with activating CD94/NKG2C NK cell receptors. Immunity 8:693–701
Ljunggren HG, Karre K (1990) In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today 11:237–244
Long EO (1999) Regulation of immune responses through inhibitory receptors. Ann Rev Immunol 17:875–904
Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, Biassoni R, Moretta L (2001) Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Ann Rev Immunol 19:197–223
Moser C, Schmidbauer C, Gurtler U, Gross C, Gehrmann M, Thonigs G, Pfister K, Multhoff G (2002) Inhibition of tumor growth in mice with severe combined immunodeficiency is mediated by heat shock protein 70 (HSP70)-peptide-activated, CD94 positive natural killer cells. Cell Stress Chaperones 7:365–373
Multhoff G, Botzler C, Wiesnet M, Eissner G, Issels R (1995a) CD3− large granular lymphocytes recognize a heat-inducible immunogenic determinant associated with the 72-kD heat shock protein on human sarcoma cells. Blood 86:1374–1382
Multhoff G, Botzler C, Wiesnet M, Muller E, Meier T, Wilmanns W, Issels RD (1995b) A stress-inducible 72-kDa heat-shock protein (HSP72) is expressed on the surface of human tumor cells, but not on normal cells. Int J Cancer 61:272–279
Multhoff G, Botzler C, Jennen L, Schmidt J, Ellwart J, Issels R (1997) Heat shock protein 72 on tumor cells: a recognition structure for natural killer cells. J Immunol 158:4341–4350
Multhoff G, Mizzen L, Winchester CC et al (1999) Heat shock protein 70 (Hsp70) stimulates proliferation and cytolytic activity of natural killer cells. Exp Hematol 27:1627–1636
Multhoff G, Pfister K, Botzler C, Jordan A, Scholz R, Schmetzer H, Burgstahler R, Hiddemann W (2000) Adoptive transfer of human natural killer cells in mice with severe combined immunodeficiency inhibits growth of Hsp70-expressing tumors. Int J Cancer 88:791–797
Multhoff G, Pfister K, Gehrmann M, Hantschel M, Gross C, Hafner M, Hiddemann W (2001) A 14-mer Hsp70 peptide stimulates natural killer (NK) cell activity. Cell Stress Chaperones 6:337–344
Radons J, Stangl S, Multhoff G (2006) NK cell-based immunotherapies against tumors. Central Eur J Med 13:170–204
Rosenberg SA, Yang JC, White DE, Steinberg SM (1998) Durability of complete responses in patients with metastatic cancer treated with high-dose interleukin-2: identification of the antigens mediating response. Ann Surg 228:307–319
Stangl S, Wortmann A, Guertler U, Multhoff G (2006) Control of metastasized pancreatic carcinomas in SCID/beige mice with human IL-2/TKD-activated NK cells. J Immunol 176:6270–6276
Ulbrecht M, Martinozzi S, Grzeschik M, Hengel H, Ellwart JW, Pla M, Weiss EH (2000) Cutting edge: the human cytomegalovirus UL40 gene product contains a ligand for HLA-E and prevents NK cell-mediated lysis. J Immunol 164:5019–5022
Whiteside TL, Vujanovic NL, Herberman RB (1998) Natural killer cells and tumor therapy. Curr Top Microbiol Immunol 230:221–244
Acknowledgments
The authors thank Professor Elisabeth Weiss (Institute of Anthropology and Human Genetics, University Ludwigs Maximilians Universität, Munich, Germany) for providing the HLA-E expressing K562 transfectants and Professor Eric Long (National Institute for Allergy and Infectious Diseases, USA) for constructive and helpful suggestions. This work was supported by EU-TRANSNET (MRTN 2004 512253), EU (LSHB-CT-2007-037703), the Bundesministerium für Bildung und Forschung (BMBF, project BioChance Plus), Deutsche Forschungsgemeinschaft (DFG, MU1238 7/2), and multimmune GmbH Munich, Germany.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stangl, S., Gross, C., Pockley, A.G. et al. Influence of Hsp70 and HLA-E on the killing of leukemic blasts by cytokine/Hsp70 peptide-activated human natural killer (NK) cells. Cell Stress and Chaperones 13, 221–230 (2008). https://doi.org/10.1007/s12192-007-0008-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-007-0008-y