Abstract
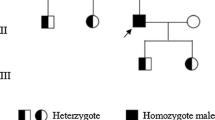
Factor X (FX) deficiency is an inherited autosomal recessive bleeding disorder. Here, we analyzed a proband with FX deficiency in a Chinese family. Genetic analysis revealed that the proband and his affected sister was homozygous for c.1085G>A mutation, corresponding to a Ser362Asn substitution. In vitro expression experiments showed that the FX Ser362Asn mutation led to a significant reduction in activity levels in the culture medium. This Ser to Asn substitution may change the shape of the active site. Moreover, simulations of molecular dynamics indicated that the binding energy of the FX Ser362Asn to the substrate is higher than that of wild type and the side-chain conformation of the catalytic residue His276 (His42) is changed. This impairs the conformational switch of the protein from zymogen to proteinase, thus causing the functional defect of FX protein. Our findings suggest that the Ser362Asn substitution is a pathogenic mutation that causes inherited FX deficiency.








Similar content being viewed by others
References
Reddy SV, Zhou ZQ, Rao KJ, Scott JP, Watzke H, High KA, et al. Molecular characterization of human factor XSan Antonio. Blood. 1989;74:1486–90.
Karimi M, Menegatti M, Afrasiabi A, Sarikhani S, Peyvandi F. Phenotype and genotype report on homozygous and heterozygous patients with congenital factor X deficiency. Haematol J. 2008;93:934–8.
Epcacan S, Menegatti M, Akbayram S, Cairo A, Peyvandi F, Oner AF. Frequency of the p.Gly262Asp mutation in congenital factor X deficiency. Eur J Clin Investig. 2015;45:1087–91.
Millar DS, Elliston L, Deex P, Krawczak M, Wacey AI, Reynaud J, et al. Molecular analysis of the genotype–phenotype relationship in factor X deficiency. Hum Genet. 2000;106:249–57.
Leytus SP, Chung DW, Kisiel W, Kurachi K, Davie EW. Characterization of a cDNA coding for human factor X. PNAS. 1984;81:3699–702.
Peyvandi F, Duga S, Akhavan S, Mannucci PM. Rare coagulation deficiencies. Haemophilia. 2002;8:308–21.
Gilgenkrantz S, Briquel ME, Andre E, Alexandre P, Jalbert P, Le Marec B, et al. Structural genes of coagulation factors VII and X located on 13q34. Ann Genet. 1986;29:32–5.
Leytus SP, Foster DC, Kurachi K, Davie EW. Gene for human factor X: a blood coagulation factor whose gene organization is essentially identical with that of factor IX and protein C. Biochemistry. 1986;25:5098–102.
Venkateswarlu D, Perera L, Darden T, Pedersen LG. Structure and dynamics of zymogen human blood coagulation factor X. Biophys J. 2002;82:1190–206.
Nagaya S, Akiyama M, Murakami M, Sekiya A, Asakura H, Morishita E. Congenital coagulation factor X deficiency: genetic analysis of five patients and functional characterization of mutant factor X proteins. Haemophilia. 2018;24:774–85.
Uprichard J, Perry DJ. Factor X deficiency. Blood Rev. 2002;16:97–110.
Wang WB, Fu QH, Zhou RF, Wu WM, Ding QL, Hu YQ, et al. Molecular characterization of two novel mutations causing factor X deficiency in a Chinese pedigree. Haemophilia. 2005;11:31–7.
Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–9.
Vaser R, Adusumalli S, Leng SN, Sikic M, Ng PC. SIFT missense predictions for genomes. Nat Protoc. 2016;11:1–9.
Katz BA, Mackman R, Luong C, Radika K, Martelli A, Sprengeler PA, et al. Structural basis for selectivity of a small molecule, S1-binding, submicromolar inhibitor of urokinase-type plasminogen activator. Chem Biol. 2000;7:299–312.
Martin ACR. Mapping PDB chains to UniProtKB entries. Bioinformatics. 2005;21:4297–301.
DeLano WL. The PyMOL Molecular Graphics System (DeLano Scientific LLC, San Carlos, CA). PyMOL molecular graphics system on World Wide Web. https://www.pymol.org. 2002.
Abdel-Azeim S, Oliva R, Chermak E, De Cristofaro R, Cavallo L. Molecular dynamics characterization of five pathogenic Factor X mutants associated with decreased catalytic activity. Biochemistry. 2014;53:6992–7001.
Jorgensen WL, Duffy EM, Tirado-Rives J. Computational investigations of protein denaturation: apomyoglobin and chaotrope–arene interactions. Philos Trans R Soc B Biol Sci. 1993;345:87–96.
Menegatti M, Vangone A, Palla R, Milano G, Cavallo L, Oliva R, et al. A recurrent Gly43Asp substitution in coagulation Factor X rigidifies its catalytic pocket and impairs catalytic activity and intracellular trafficking. Thromb Res. 2014;133:481–87.
Girolami A, Ferrari S, Cosi E, Santarossa C, Randi ML. Vitamin K-dependent coagulation factors that may be responsible for both bleeding and thrombosis (FII, FVII, and FIX). Clin Appl Thromb Hemost. 2018;24:42S–7S.
Herrmann F, Auerswald G, Ruiz-saez A, Navarrete M, Pollmann H, Lopaciuk S, et al. Factor X deficiency: clinical manifestation of 102 subjects from Europe and Latin America with mutations in the factor 10 gene. Haemophilia. 2006;12:479–89.
Girolami A, Vettore S, Scarparo P, Lombardi AM. Persistent validity of a classification of congenital factor X defects based on clotting, chromogenic and immunological assays even in the molecular biology era. Haemophilia. 2011:17:17–20.
Sun N, Chen Y, Peng H, Luo Y, Zhang G. A novel Ala275Val mutation in factor X gene influences its structural compatibility and impairs intracellular trafficking and coagulant activity. Thromb Res. 2016: 138:108–13.
Tartary M, Vidaud D, Piao Y, Costa JM, Bahnak BR, Fressinaud E, et al. Detection of a molecular defect in 40 of 44 patients with haemophilia B by PCR and denaturing gradient gel electrophoresis. Br J Haematol. 1993:84:662–69.
Acknowledgements
We thank the family for their participation in this research.
Funding
This work was supported in part by the National Natural Science Foundation, PR China (nos. 81970172, 81700182). This research was also supported by Shanxi Provincial Key Research and Development Project (no. 201803D31123) and the Natural Science Foundation of Shanxi Province (201601D202094).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests, financial or non-financial.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Zhang, X., Chen, K., Wang, G. et al. Molecular mechanism of a novel Ser362Asn mutation causing inherited FX deficiency in a Chinese family. Int J Hematol 112, 8–16 (2020). https://doi.org/10.1007/s12185-020-02877-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-020-02877-y