Abstract
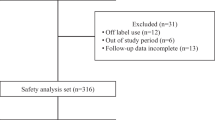
This retrospective study analyzes the results of radioimmunotherapy (RIT) with 90Y-ibritumomab tiuxetan in 94 Japanese patients with relapsed or refractory low-grade B cell non-Hodgkin lymphoma at a single institution. All patients had previously been administered with 1–8 (median 1) regimens of rituximab alone or combined with other chemotherapeutic regimens at a mean age of 64 years. The overall response rate was 90 % and the complete response (CR) rate was 69 %. The median overall survival was not reached and progression-free survival (PFS) was 26 months, respectively, for the early phase 50 patients during a median follow-up period of 46.5 months. In this cohort, the PFS rates for the 50 early phase patients who had undergone ≤2 and ≥3 previous regimens, and for those who achieved CR compared with those who did not (partial response, PR; stable disease, SD; progressive disease, PD) were 38 and 11 months, respectively. Multivariate analysis showed that these two factors were statistically significant (p = 0.0011 and p <0.0001, respectively). The overall incidence of grade ≥3 non-hematological toxicity was 9 %. Two patients died of treatment-related deteriorating hepatitis C. A second malignancy developed in two patients at 10.5 and 3.5 months after treatment. We recommend administering 90Y-ibritumomab tiuxetan as early in the disease course as possible, and at the latest as a third-line therapy to maximize the benefits of RIT, which should improve the quality of life for patients.






Similar content being viewed by others
References
Witzig TE, Vukov AM, Habermann TM, Geyer S, Kurtin PJ, Friedenberg WR, et al. Rituximab therapy for patients with newly diagnosed, advanced-stage, follicular grade I non-Hodgkin’s lymphoma: a phase II trial in the North Central Cancer Treatment Group. J Clin Oncol. 2005;23:1103–8.
Colombat PL, Salles G, Brousse N, Eftekhari P, Soubeyran P, Delwail V, et al. Rituximab (anti-CD20 monoclonal antibody) as single first-line therapy for patients with follicular lymphoma with a low tumor burden: clinical and molecular evaluation. Blood. 2001;97:101–6.
Hiddemann W, Kneba M, Dreyling M, Schmitz N, Lengfelder E, Schmits R, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the german low-grade lymphoma study group. Blood. 2005;106:3725–32.
Marcus R, Imrie K, Belch A, Cunningham D, Flores E, Catalano J, et al. CVP chemotherapy plus rituximab compared with CVP as first-line treatment for advanced follicular lymphoma. Blood. 2005;105:1417–23.
Witzig TE, White CA, Wiseman GA, Gordon LI, Emmanouilides C, Raubitschek A, et al. Phase I/II trial of IDEC-Y2B8 radioimmunotherapy for treatment of relapsed or refractory CD20(+) B-cell non-Hodgkin’s lymphoma. J Clin Oncol. 1999;17:3793–803.
Morschhauser F, Radford J, Van Hoof A, Botto B, Rohatiner AZ, Salles G, et al. 90Yttrium-ibritumomab tiuxetan consolidation of first remission in advanced-stage follicular non-Hodgkin lymphoma: updated results after a median follow-up of 7.3 years from the international, randomized, phase III first-line indolent trial. J Clin Oncol. 2013;31:1977–83.
Witzig TE, Flinn IW, Gordon LI, Emmanouilides C, Czuczman MS, Saleh MN, et al. Treatment with ibritumomab tiuxetan radioimmunotherapy in patients with rituximab-refractory follicular non-Hodgkin’s lymphoma. J Clin Oncol. 2002;20:3262–9.
Witzig TE, White CA, Gordon LI, Wiseman GA, Emmanoulides C, Murray JL, et al. Safety of Yttrium-90 ibritumomab tiuxetan radioimmunotherapy for relapsed low-grade, follicular, or transformed non-Hodgkin’s lymphoma. J Clin Oncol. 2003;21:1263–70.
Watanabe T, Terui S, Itoh K, Terauchi T, Igarashi T, Usubuchi N, et al. Phase I study of radioimmunotherapy with an anti-CD20 murine radioimmunoconjugate ((90)Y-ibritumomab tiuxetan) in relapsed or refractory indolent B-cell lymphoma. Cancer Sci. 2005;96:903–10.
Tobinai K, Watanabe T, Ogura M, Morishima Y, Hotta T, Ishizawa K, et al. Japanese phase II study of 90Y-ibritumomab tiuxetan in patients with relapsed or refractory indolent B-cell lymphoma. Cancer Sci. 2009;100:158–64.
Solal-Céligny P, Roy P, Colombat P, White J, Armitage JO, Arranz-Saez R, et al. Follicular lymphoma international prognostic index. Blood. 2004;104:1258–65.
Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. International harmonization project on lymphoma. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–86.
US National Institutes of Health. CTCAE/CTC Archive. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_archive
Seiler TM, Hiddemann W. Advances in the management of follicular lymphoma. Curr Opin Oncol. 2012;24:742–7.
Witzig TE, Molina A, Gordon LI, Emmanouilides C, Schider RJ, Flinn IW, et al. Long-term responses in patients with recurring or refractory B-cell non-Hodgkin lymphoma treated with yttrium 90 ibritumomab tiuxetan. Cancer. 2007;109:1804–10.
Lopci E, Santi I, Derenzini E, Fonti C, Savelli G, Bertagna F, et al. FDG-PET in the assessment of patients with follicular lymphoma treated by ibritumomab tiuxetan Y 90: multicentric study. Ann Oncol. 2010;21:1877–83.
Guidetti A, Carlo-Stella C, Ruella M, Miceli R, Devizzi L, Locatelli SL, et al. Myeloablative doses of Yttrium-90-Ibritumomab tiuxetan and the risk of secondary myelodysplasia/acute myelogenous leukemia. Cancer. 2011;117:5074–84.
Czuczman MS, Emmanouilides C, Darif M, Witzig TE, Gordon LI, Revell S, et al. Treatment-related myelodysplastic syndrome and acute myelogenous leukemia in patients treated with ibritumomab tiuxetan radioimmunotherapy. J Clin Oncol. 2007;25:4285–92.
Scholz CW, Pinto A, Linkesch W, Lindén O, Viardot A, Keller U, et al. 90Yttrium-Ibritumomab-Tiuxetan as first-line treatment for follicular lymphoma: 30 months of follow-up data from an international multicenter phase II clinical trial. J Clin Oncol. 2013;31:308–13.
Illidge TM, Mayes S, Pettengell R, Bates AT, Bayne M, Radford JA, et al. Fractionated 90Y-Ibritumomab Tiuxetan radioimmunotherapy as an Initial therapy of follicular lymphoma: an international phase II study in patients requiring treatment according to GELF/BNLI criteria. J Clin Oncol. 2014;32:212–8.
Decaudin D, Mounier N, Tilly H, Ribrag V, Ghesqiéres H, Bouabdallah K, et al. (90)Y ibritumomab tiuxetan (Zevalin) combined with BEAM (A-BEAM) conditioning regimen plus autologous stem cell transplantation in relapsed or refractory low-grade CD20-positive B-cell lymphoma. A GELA phase II prospective study. Clin Lymphoma Myeloma Leuk. 2011;12:212–8.
Yang DH, Kim WS, Kim SJ, Kim JS, Kwak JY, Chung JS, et al. Pilot trial of yttrium-90 ibritumomab tiuxetan consolidation following rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone chemotherapy in patients with limited-stage, bulky diffuse large B-cell lymphoma. Leuk Lymphoma. 2012;53:807–11.
Briones J, Novellii S, García-Marco JA, Tomás JF, Bernal T, Gramde C, et al. Autologous stem cell transplantation after conditioning with yttrium-90 ibritumomab tiuxetan plus BEAM in refractory non-Hodgkin diffuse large B-cell lymphoma: results of a prospective, multicenter, phase II clinical trial. Haematology. 2014;99:505–10.
Gopal AK, Guthrie KA, Rajendran J, Pagel JM, Oliveira G, Maloney DG, et al. 90Y-ibritumomab tiuxetan, fludarabine, and TBI-based nonmyeloablative allogeneic transplantation conditioning for patients with persistent high-risk B-cell lymphoma. Blood. 2011;118:1132–9.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Uike, N., Choi, I., Tsuda, M. et al. Factors associated with effects of 90Y-ibritumomab tiuxetan in patients with relapsed or refractory low-grade B cell non-Hodgkin lymphoma: single-institution experience with 94 Japanese patients in rituximab era. Int J Hematol 100, 386–392 (2014). https://doi.org/10.1007/s12185-014-1636-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-014-1636-5