Abstract
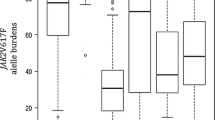
JAK2V617F, a gain-of-function mutation in the tyrosine kinase JAK2, is frequently detected in classical myeloproliferative neoplasms (MPNs). In the present study, we determined the JAK2V617F allele burden in Japanese MPN patients using alternately binding probe competitive-polymerase chain reaction, a highly quantitative method recently developed by our group. Although we observed strong similarities in terms of epidemiological parameters associated with the JAK2V617F allele burden between our cohort and others, we found a higher JAK2V617F allele burden in Japanese polycythemia vera (PV) patients and lower frequencies of thrombosis in Japanese MPN patients compared with previous reports. In addition, despite the presence of high red blood cell counts, some patients bearing the JAK2V617F mutation were not diagnosed as PV, as their hemoglobin values were lower than the WHO PV criterion. In these patients, the JAK2V617F allele burden was strikingly similar to that in PV patients fulfilling the 2008 WHO criteria, suggesting that these patients can be classified as PV. Although isotopic measurement of red cell mass (RCM) is required for definitive diagnosis of PV, our data suggest that precise measurement of the JAK2V617F allele burden may improve the diagnosis of PV when RCM has not been determined.




Similar content being viewed by others
References
Tefferi A. Novel mutations and their functional and clinical relevance in myeloproliferative neoplasms: JAK2, MPL, TET2, ASXL1, CBL, IDH and IKZF1. Leukemia. 2010;24:1128–38.
Milosevic JD, Kralovics R. Genetic and epigenetic alterations of myeloproliferative disorders. Int J Hematol. 2013;97:183–97.
Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–61.
James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–8.
Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–90.
Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–97.
Jones AV, Kreil S, Zoi K, Waghorn K, Curtis C, Zhang L, et al. Widespread occurrence of the JAK2 V617F mutation in chronic myeloproliferative disorders. Blood. 2005;106:2162–8.
Vizmanos JL, Ormazabal C, Larrayoz MJ, Cross NC, Calasanz MJ. JAK2 V617F mutation in classic chronic myeloproliferative diseases: a report on a series of 349 patients. Leukemia. 2006;20:534–5.
Larsen TS, Pallisgaard N, Moller MB, Hasselbalch HC. The JAK2 V617F allele burden in essential thrombocythemia, polycythemia vera and primary myelofibrosis—impact on disease phenotype. Eur J Haematol. 2007;79:508–15.
Hussein K, Bock O, Theophile K, von Neuhoff N, Buhr T, Schlue J, et al. JAK2(V617F) allele burden discriminates essential thrombocythemia from a subset of prefibrotic-stage primary myelofibrosis. Exp Hematol. 2009;37:1186–93.
Akada H, Yan D, Zou H, Fiering S, Hutchison RE, Mohi MG. Conditional expression of heterozygous or homozygous Jak2V617F from its endogenous promoter induces a polycythemia vera-like disease. Blood. 2010;115:3589–97.
Xing S, Wanting TH, Zhao W, Ma J, Wang S, Xu X, et al. Transgenic expression of JAK2V617F causes myeloproliferative disorders in mice. Blood. 2008;111:5109–17.
Shide K, Shimoda HK, Kumano T, Karube K, Kameda T, Takenaka K, et al. Development of ET, primary myelofibrosis and PV in mice expressing JAK2 V617F. Leukemia. 2008;22:87–95.
Marty C, Lacout C, Martin A, Hasan S, Jacquot S, Birling MC, et al. Myeloproliferative neoplasm induced by constitutive expression of JAK2V617F in knock-in mice. Blood. 2010;116:783–7.
Tiedt R, Hao-Shen H, Sobas MA, Looser R, Dirnhofer S, Schwaller J, et al. Ratio of mutant JAK2-V617F to wild-type Jak2 determines the MPD phenotypes in transgenic mice. Blood. 2008;111:3931–40.
Li J, Spensberger D, Ahn JS, Anand S, Beer PA, Ghevaert C, et al. JAK2 V617F impairs hematopoietic stem cell function in a conditional knock-in mouse model of JAK2 V617F-positive essential thrombocythemia. Blood. 2010;116:1528–38.
Tefferi A, Thiele J, Orazi A, Kvasnicka HM, Barbui T, Hanson CA, et al. Proposals and rationale for revision of the World Health Organization diagnostic criteria for polycythemia vera, essential thrombocythemia, and primary myelotibrosis: recommendations from an ad hoc international expert panel. Blood. 2007;110:1092–7.
Campbell PJ, Scott LM, Buck G, Wheatley K, East CL, Marsden JT, et al. Definition of subtypes of essential thrombocythaemia and relation to polycythaemia vera based on JAK2 V617F mutation status: a prospective study. Lancet. 2005;366:1945–53.
Vannucchi AM, Antonioli E, Guglielmelli P, Longo G, Pancrazzi A, Ponziani V, et al. Prospective identification of high-risk polycythemia vera patients based on JAK2(V617F) allele burden. Leukemia. 2007;21:1952–9.
Vannucchi AM, Antonioli E, Guglielmelli P, Pardanani A, Tefferi A. Clinical correlates of JAK2V617F presence or allele burden in myeloproliferative neoplasms: a critical reappraisal. Leukemia. 2008;22:1299–307.
Antonioli E, Guglielmelli P, Poli G, Bogani C, Pancrazzi A, Longo G, et al. Influence of JAK2(V617F) allele burden on phenotype in essential thrombocythemia. Haematologica. 2008;93:41–8.
Carobbio A, Finazzi G, Antonioli E, Guglielmelli P, Vannucchi AM, Dellacasa CM, et al. JAK2V617F allele burden and thrombosis: a direct comparison in essential thrombocythemia and polycythemia vera. Exp Hematol. 2009;37:1016–21.
Passamonti F, Rumi E, Pietra D, Elena C, Boveri E, Arcaini L, et al. A prospective study of 338 patients with polycythemia vera: the impact of JAK2 (V617F) allele burden and leukocytosis on fibrotic or leukemic disease transformation and vascular complications. Leukemia. 2010;24:1574–9.
Silver RT, Vandris K, Wang YL, Adriano F, Jones AV, Christos PJ, et al. JAK2(V617F) allele burden in polycythemia vera correlates with grade of myelofibrosis, but is not substantially affected by therapy. Leuk Res. 2011;35:177–82.
Guglielmelli P, Barosi G, Specchia G, Rambaldi A, Lo Coco F, Antonioli E, et al. Identification of patients with poorer survival in primary myelofibrosis based on the burden of JAK2V617F mutated allele. Blood. 2009;114:1477–83.
Ha JS, Kim YK, Jung SI, Jung HR, Chung IS. Correlations between Janus kinase 2 V617F allele burdens and clinicohematologic parameters in myeloproliferative neoplasms. Ann Lab Med. 2012;32:385–91.
Zhou J, Ye YX, Zeng SG, Zhou Y, Mao ZG, Song XB, et al. Impact of JAK2 V617F mutation on hemogram variation in patients with non-reactive elevated platelet counts. PLoS One. 2013;8:e57856.
Park SH, Chi HS, Cho YU, Jang S, Park CJ. The allele burden of JAK2 V617F can aid in differential diagnosis of Philadelphia chromosome-negative myeloproliferative neoplasm. Blood Res. 2013;48:128–32.
Morishita S, Komatsu N, Kirito K, Koda AH, Sekiguchi Y, Tsuneda S, et al. Alternately binding probe competitive PCR as a simple, cost-effective, and accurate quantification method for JAK2V617F allele burden in myeloproliferative neoplasms. Leuk Res. 2011;35:1632–6.
Rapado I, Albizua E, Ayala R, Hernandez JA, Garcia-Alonso L, Grande S, et al. Validity test study of JAK2 V617F and allele burden quantification in the diagnosis of myeloproliferative diseases. Ann Hematol. 2008;87:741–9.
Dan K, Yamada T, Kimura Y, Usui N, Okamoto S, Sugihara T, et al. Clinical features of polycythemia vera and essential thrombocythemia in Japan: retrospective analysis of a nationwide survey by the Japanese Elderly Leukemia and Lymphoma Study Group. Int J Hematol. 2006;83:443–9.
Finazzi G, Caruso V, Marchioli R, Capnist G, Chisesi T, Finelli C, et al. Acute leukemia in polycythemia vera: an analysis of 1638 patients enrolled in a prospective observational study. Blood. 2005;105:2664–70.
Kittur J, Knudson RA, Lasho TL, Finke CM, Gangat N, Wolanskyj AP, et al. Clinical correlates of JAK2V617F allele burden in essential thrombocythemia. Cancer. 2007;109:2279–84.
Tefferi A, Elliott M. Thrombosis in myeloproliferative disorders: prevalence, prognostic factors, and the role of leukocytes and JAK2V617F. Semin Thromb Hemost. 2007;33:313–20.
Gorog DA, Yamamoto J, Saraf S, Ishii H, Ijiri Y, Ikarugi H, et al. First direct comparison of platelet reactivity and thrombolytic status between Japanese and Western volunteers: possible relationship to the “Japanese paradox”. Int J Cardiol. 2011;152:43–8.
Scott LM. The JAK2 exon 12 mutations: a comprehensive review. Am J Hematol. 2011;86:668–76.
Schnittger S, Bacher U, Haferlach C, Geer T, Muller P, Mittermuller J, et al. Detection of JAK2 exon 12 mutations in 15 patients with JAK2V617F negative polycythemia vera. Haematologica. 2009;94:414–8.
Moliterno AR, Williams DM, Rogers O, Spivak JL. Molecular mimicry in the chronic myeloproliferative disorders: reciprocity between quantitative JAK2 V617F and Mpl expression. Blood. 2006;108:3913–5.
Lippert E, Boissinot M, Kralovics R, Girodon F, Dobo I, Praloran V, et al. The JAK2-V617F mutation is frequently present at diagnosis in patients with essential thrombocythemia and polycythemia vera. Blood. 2006;108:1865–7.
Okoli S, Harrison C. Emerging treatments for essential thrombocythemia. J Blood Med. 2011;2:151–9.
Briere JB. Essential thrombocythemia. Orphanet J Rare Dis. 2007;2:3.
Barbui T, Thiele J, Carobbio A, Gisslinger H, Finazzi G, Rumi E, et al. Masked polycythemia vera diagnosed according to WHO and BCSH classification. Am J Hematol. 2014;89(2):199–202.
Johansson PL, Safai-Kutti S, Kutti J. An elevated venous haemoglobin concentration cannot be used as a surrogate marker for absolute erythrocytosis: a study of patients with polycythaemia vera and apparent polycythaemia. Br J Haematol. 2005;129:701–5.
Silver RT, Chow W, Orazi A, Arles SP, Goldsmith SJ. Evaluation of WHO criteria for diagnosis of polycythemia vera: a prospective analysis. Blood. 2013;122:1881–6.
Alvarez-Larran A, Ancochea A, Angona A, Pedro C, Garcia-Pallarols F, Martinez-Aviles L, et al. Red cell mass measurement in patients with clinically suspected diagnosis of polycythemia vera or essential thrombocythemia. Haematologica. 2012;97:1704–7.
McMullin MF, Reilly JT, Campbell P, Bareford D, Green AR, Harrison CN, et al. Amendment to the guideline for diagnosis and investigation of polycythaemia/erythrocytosis. Br J Haematol. 2007;138:821–2.
Acknowledgments
We thank Kazuhiko Ikeda (Fukushima Medical University), Nobuyoshi Hanaoka (Wakayama Medical University), Toshiro Kurokawa (Toyama Red Cross Hospital), Hideo Harigae (Tohoku University), Takayuki Ikezoe (Kochi University), Jun Murakami (University of Toyama), Kensuke Usuki (NTT Kanto Medical Center), and Takao Hirano (Juntendo Nerima Hospital) for providing patient specimens and clinical information. We also thank Masaki Kobayashi and Ei Leen Liew for technical assistance and fruitful discussions; Yuki Kagawa for advice on statistics; Kyoko Kubo, Kazuko Kawamura, and Megumi Hasegawa for secretarial assistance; and other members of the Department of Hematology for support in this study. We also acknowledge the Laboratory of Molecular and Biochemical Research, Research Support Center, Juntendo University Graduate School of Medicine. This work was funded in part by JSPS (http://www.jsps.go.jp/english/e-grants/) KAKENHI Grant #25860416 (SM). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare no competing interests.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Edahiro, Y., Morishita, S., Takahashi, K. et al. JAK2V617F mutation status and allele burden in classical Ph-negative myeloproliferative neoplasms in Japan. Int J Hematol 99, 625–634 (2014). https://doi.org/10.1007/s12185-014-1567-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-014-1567-1