Abstract
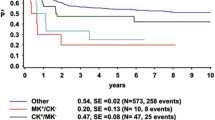
Karyotype analysis in acute myeloid leukemia (AML) is one of the powerful prognostic factors for complete remission (CR), relapse, and overall survival (OS). Cytogenetic mosaicism is considered to be one of the important characteristics in expression of phenotypic manifestations. However, it has not come into focus due to emerging molecular biological approaches and the results of a number of mutation studies. Clinical correlates and prognostic relevance of mosaicism were evaluated in 163 AML patients [adverse-risk karyotypes (n = 72) and undefined karyotypes (n = 91)]. All patients were treated by induction and consolidation chemotherapies and finally went on hematopoietic stem cell transplantations (HSCT). Patients were divided into two subgroups, either with or without normal karyotype (NK) mosaicism. Seventy patients exhibited NK mosaicism and 93 did not. There were no significant differences in age, gender, chemotherapy cycles to achieve CR, HSCT donor type, source or intensity properties between the two subgroups. We found that NK mosaicism remaining in adverse-risk and undefined karyotype at diagnosis significantly correlates with better OS (p = 0.001) and lower CIR (p = 0.021) rate after HSCT. Our data show that the poor prognostic properties of unfavorable risk karyotype can be overcome to a great extent by allogeneic HSCT and chronic GVHD, especially in the subgroup with NK mosaicism. Cytogenetic mosaicism at initial diagnosis can be an influential factor for survival outcomes, even after HSCT.



Similar content being viewed by others
References
Bacher U, Kern W, Alpermann T, et al. Prognosis in patients with MDS or AML and bone marrow blasts between 10% and 30% is not associated with blast counts but depends on cytogenetic and molecular genetic characteristics. Leukemia. 2011;25:1361–4.
Grimwade D, Hills RK, Moorman AV, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116:354–65.
Byrd JC, Mrozek K, Dodge RK, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461). Blood. 2002;100:4325–36.
Grimwade D, Walker H, Oliver F, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children’s Leukaemia Working Parties. Blood. 1998;92:2322–33.
Youssoufian H, Pyeritz RE. Mechanisms and consequences of somatic mosaicism in humans. Nat Rev Genet. 2002;3:748–58.
Ballif BC, Rorem EA, Sundin K, et al. Detection of low-level mosaicism by array CGH in routine diagnostic specimens. Am J Med Genet A. 2006;140:2757–67.
Leon E, Zou YS, Milunsky JM. Mosaic down syndrome in a patient with low-level mosaicism detected by microarray. Am J Med Genet Part A. 2010;152A:3154–6.
Shin M, Siffel C, Correa A. Survival of children with mosaic Down syndrome. Am J Med Genet A. 2010;152A:800–1.
Landstrom AP, Knudson RA, Dewald GW, Ketterling RP, Tefferi A. Philadelphia chromosome mosaicism at diagnosis in chronic myeloid leukemia: clinical correlates and effect on imatinib mesylate treatment outcome. Leuk Lymphoma. 2007;48:2137–40.
Golde DW, Bersch NL, Sparkes RS. Chromosomal mosaicism associated with prolonged remission in chronic myelogenous leukemia. Cancer. 1976;37:1849–52.
Sokal JE. Significance of Ph1-negative marrow cells in Ph1-positive chronic granulocytic leukemia. Blood. 1980;56:1072–6.
Estey EH, Pierce S, Keating MJ. Identification of a group of AML/MDS patients with a relatively favorable prognosis who have chromosome 5 and/or 7 abnormalities. Haematologica. 2000;85:246–9.
Medeiros BC, Othus M, Fang M, Appelbaum FR, Estey EH. Impact of residual normal metaphases in core binding factor acute myeloid leukemia. Cancer. 2012;118:2420–3.
O’Donnell MR, Abboud CN, Altman J, et al. Acute myeloid leukemia. J Natl Compr Canc Netw. 2011;9:280–317.
Park HS, Kim DW, Kim CC, et al. Induction chemotherapy with idarubicin plus N4-behenoyl-1-beta-d-arabinofuranosylcytosine in acute myelogenous leukemia: a newly designed induction regimen–a prospective, cooperative multicenter study. Semin Hematol. 1996;33:24–9.
Kim HJ, Min WS, Eom KS, et al. Autologous stem cell transplantation using modified TAM or combination of triple-alkylating agents conditioning regimens as one of the post-remission treatments in patients with adult acute myeloid leukemia in first complete remission. Bone Marrow Transplant. 2004;34:215–20.
Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–54.
Xie B, Othus M, Medeiros BC, et al. Influence of residual normal metaphases in acute myeloid leukemia patients with monosomal karyotype. Haematologica. 2011;96:631–2.
Perissel B, Benkhalifa M, Taillandier J, et al. Karyotype and FISH analysis of a newly established cell line derived from a human bladder carcinoma. Cancer Genet Cytogenet. 1993;67:101–7.
Mrozek K, Bloomfield CD. Acute myeloid leukemia with adverse cytogenetic risk. Oncology (Williston Park). 2012;26(714):723.
Schoch C, Haase D, Haferlach T, et al. Fifty-one patients with acute myeloid leukemia and translocation t(8;21)(q22;q22): an additional deletion in 9q is an adverse prognostic factor. Leukemia. 1996;10:1288–95.
Appelbaum FR, Kopecky KJ, Tallman MS, et al. The clinical spectrum of adult acute myeloid leukaemia associated with core binding factor translocations. Br J Haematol. 2006;135:165–73.
Boissel N, Leroy H, Brethon B, et al. Incidence and prognostic impact of c-Kit, FLT3, and Ras gene mutations in core binding factor acute myeloid leukemia (CBF-AML). Leukemia. 2006;20:965–70.
Park SH, Chi HS, Min SK, et al. Prognostic impact of c-KIT mutations in core binding factor acute myeloid leukemia. Leuk Res. 2011;35:1376–83.
Kim HJ, Ahn HK, Jung CW, et al. KIT D816 mutation associates with adverse outcomes in core binding factor acute myeloid leukemia, especially in the subgroup with RUNX1/RUNX1T1 rearrangement. Ann Hematol. 2013;92:163–71.
Acknowledgments
This study was supported by a Grant from the National R&D Program for Cancer Control, Ministry for Health and Welfare, Republic of Korea (1020370).
Conflict of interest
Authors declare no conflict of interest in this study.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Yoon, JH., Kim, HJ., Shin, SH. et al. Normal karyotype mosaicism in adult AML patients with adverse-risk and undefined karyotype: preliminary report of treatment outcomes after hematopoietic stem cell transplantation. Int J Hematol 97, 773–781 (2013). https://doi.org/10.1007/s12185-013-1335-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-013-1335-7