Abstract
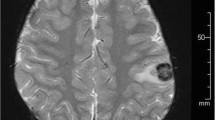
Post-transplant lymphoproliferative disorder (PTLD) is a fatal complication of allogeneic hematopoietic stem cell transplantation (HSCT) that is caused by reactivation of Epstein–Barr virus (EBV). A successful approach, monitoring EBV-DNA load in peripheral blood (PB) accompanied by preemptive rituximab therapy, has recently been reported. Here, we describe a 29-year-old woman who developed isolated central nervous system (CNS) PTLD. She received HSCT against acute myelogenous leukemia from a related human leukocyte antigen-haploidentical donor, following a conditioning regimen that included antithymocyte globulin. Tacrolimus and methylprednisolone were given as prophylaxis for graft-versus-host disease. On day +172, the patient’s consciousness deteriorated. Magnetic resonance imaging showed six ring-enhanced lesions in the cerebral hemispheres. These tumors were diagnosed, via a craniotomy and tumorectomy, as PTLD. EBV-DNA load was elevated in the cerebrospinal fluid (CSF) but not detected in PB. She was treated with whole-brain irradiation and rituximab, and achieved partial remission of the tumors. This case serves as a reminder that vigilance is required regarding the development of isolated CNS PTLD; it is worth examining EBV-DNA replication in CSF for diagnosis even when the EBV-DNA load is negative in PB.


Similar content being viewed by others
References
Shapiro RS, McClain K, Frizzera G, Gajl-Peczalska KJ, Kersey JH, Blazar BR, et al. Epstein–Barr virus associated B cell lymphoproliferative disorders following bone marrow transplantation. Blood. 1988;71(5):1234–43.
Styczynski J, Reusser P, Einsele H, de la Camara R, Cordonnier C, Ward KN, et al. Management of HSV, VZV and EBV infections in patients with hematological malignancies and after SCT: guidelines from the Second European Conference on Infections in Leukemia. Bone Marrow Transplant. 2009;43:757–70.
Curtis RE, Travis LB, Rowlings PA, Socié G, Kingma DW, Banks PM, et al. Risk of lymphoproliferative disorders after bone marrow transplantation: a multi-institutional study. Blood. 1999;94:2208–16.
Landgren O, Gilbert ES, Rizzo JD, Socié G, Banks PM, Sobocinski KA, et al. Risk factors for lymphoproliferative disorders after allogeneic hematopoietic cell transplantation. Blood. 2009;113:4992–5001.
Reddy N, Rezvani K, Barrett AJ, Savani BN. Strategies to prevent EBV reactivation and posttransplant lymphoproliferative disorders (PTLD) after allogeneic stem cell transplantation in high-risk patients. Biol Blood Marrow Transplant. 2011;17:591–7.
van Esser JW, Niesters HG, van der Holt B, Meijer E, Osterhaus AD, Gratama JW, et al. Prevention of Epstein–Barr virus-lymphoproliferative disease by molecular monitoring and preemptive rituximab in high-risk patients after allogeneic stem cell transplantation. Blood. 2002;99:4364–9.
Weinstock DM, Ambrossi GG, Brennan C, Kiehn TE, Jakubowski A. Preemptive diagnosis and treatment of Epstein–Barr virus-associated post transplant lymphoproliferative disorder after hematopoietic stem cell transplant: an approach in development. Bone Marrow Transplant. 2006;37:539–46.
Ahmad I, Cau NV, Kwan J, Maaroufi Y, Meuleman N, Aoun M, et al. Preemptive management of Epstein–Barr virus reactivation after hematopoietic stem-cell transplantation. Transplantation. 2009;87:1240–5.
Nagafuji K, Eto T, Hayashi S, Oshima K, Maeda Y, Gondo H, et al. Donor lymphocyte transfusion for the treatment of Epstein–Barr virus-associated lymphoproliferative disorder of the brain. Bone Marrow Transplant. 1998;21:1155–8.
Terasawa T, Ohashi H, Tsushita K, Utsumi M, Mukai E, Nakamura S, et al. Failure to detect Epstein–Barr virus (EBV) DNA in plasma by real-time PCR in a case of EBV-associated posttransplantation lymphoproliferative disorder confined to the central nervous system. Int J Hematol. 2002;75:416–20.
Pakakasama S, Eames GM, Morriss MC, Huls MH, Rooney CM, Heslop HE, et al. Treatment of Epstein–Barr virus lymphoproliferative disease after hematopoietic stem-cell transplantation with hydroxyurea and cytotoxic T-cell lymphocytes. Transplantation. 2004;78:755–7.
Nozzoli C, Bartolozzi B, Guidi S, Orsi A, Vannucchi AM, Leoni F, et al. Epstein–Barr virus-associated post-transplant lymphoproliferative disease with central nervous system involvement after unrelated allogeneic hematopoietic stem cell transplantation. Leuk Lymphoma. 2006;47:167–9.
Hamadani M, Martin LK, Benson DM, Copelan EA, Devine SM, Hofmeister CC. Central nervous system post-transplant lymphoproliferative disorder despite negative serum and spinal fluid Epstein–Barr virus DNA PCR. Bone Marrow Transplant. 2007;39:249–51.
Aisa Y, Mori T, Nakazato T, Suzuki S, Suzuki N, Ikeda Y, et al. Primary central nervous system post-transplant lymphoproliferative disorder presenting as cerebral hemorrhage after unrelated bone marrow transplantation. Transpl Infect Dis. 2009;11:438–41.
Vu T, Carrum G, Hutton G, Heslop HE, Brenner MK, Kamble R. Human herpesvirus-6 encephalitis following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2007;39:705–9.
Heslop HE, Slobod KS, Pule MA, Hale GA, Rousseau A, Smith CA, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115:925–35.
van Esser JW, van der Holt B, Meijer E, Niesters HG, Trenschel R, Thijsen SF, et al. Epstein–Barr virus (EBV) reactivation is a frequent event after allogeneic stem cell transplantation (SCT) and quantitatively predicts EBV-lymphoproliferative disease following T-cell-depleted SCT. Blood. 2001;98:972–8.
Stevens SJ, Verschuuren EA, Pronk I, van Der Bij W, Harmsen MC, The TH, et al. Frequent monitoring of Epstein–Barr virus DNA load in unfractionated whole blood is essential for early detection of posttransplant lymphoproliferative disease in high-risk patients. Blood. 2001;97:1165–71.
Harjunpää A, Wiklund T, Collan J, Janes R, Rosenberg J, Lee D, et al. Complement activation in circulation and central nervous system after rituximab (anti-CD20) treatment of B-cell lymphoma. Leuk Lymphoma. 2001;42:731–8.
Muldoon LL, Lewin SJ, Dósa E, Kraemer DF, Pagel MA, Doolittle ND, et al. Imaging and therapy with rituximab anti-CD20 immunotherapy in an animal model of central nervous system lymphoma. Clin Cancer Res. 2011;17:2207–15.
Conflict of interest
The authors declare no competing financial interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Shimizu, H., Saitoh, T., Koya, H. et al. Discrepancy in EBV-DNA load between peripheral blood and cerebrospinal fluid in a patient with isolated CNS post-transplant lymphoproliferative disorder. Int J Hematol 94, 495–498 (2011). https://doi.org/10.1007/s12185-011-0951-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-011-0951-3