Abstract
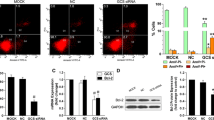
Previous work from our laboratory demonstrated that glucosylceramide synthase (GCS) and multidrug resistance 1 gene (MDR1) are co-overexpressed in drug-resistant leukemia cells. We hypothesized that GCS and MDR1 may interact. In this study, we used RNA interference (RNAi) to silence the GCS or MDR1 gene in K562/AO2 drug-resistant cells. The sensitivity of cells to different treatments with doxorubicin was evaluated. We used Taqman probe fluorescence real-time quantitative PCR, and detected expression of GCS and MDR1 mRNAs in different interfering groups. Intracellular mean fluorescence intensity (MFI), which represents rhodamine123 (rh123) retention, was determined by flow cytometry (FCM). An MTT cytotoxicity assay showed that the 50% inhibition concentration (IC50) of doxorubicin of K562/AO2 cells (138.25 ± 3.75 µg/ml) was significantly higher than that of K562 drug-sensitive cells (2.125 ± 0.125 µg/ml), and that IC50 was evidently lower in K562/AO2 cells, whether it was transfected with a small interfering RNA (siRNA) targeting GCS (GCSsiRNA) or one targeting MDR1 (MDR1siRNA). Compared with untreated K562/AO2 cells, the inhibition rates of GCS mRNA in the cells transfected with GCSsiRNA for 9 and 36 h were 56.67 ± 9.29% (p < 0.05) and 74 ± 6.38% (p < 0.05), respectively. Interestingly, the expression of MDR1 mRNA was also inhibited to 51.7 ± 4.5% (p < 0.05) 36 h after transfection with GCSsiRNA, but there was no significant difference in MDR1 expression at 9 h post-transfection in cells treated with GCSsiRNA and a negative control. It is well known that rh123 retention in cells results from an efflux function of P-glycoprotein (P-gp). In K562 cells, rh123 retention was much higher than in K562/AO2 cells (p < 0.01). We also noted that rh123 retention in the K562/AO2 cells transfected with GCSsiRNA for 48 h was significantly higher than in the negative control group. In conclusion, we show in the present study that inhibition of the GCS gene affects the expression of MDR1 mRNA and P-gp function.







Similar content being viewed by others
References
Friedenberg WR, Tallman MS, Brodsky I, Paietta E, Rowe JM, Lee SJ, Rowland Jr KM, Schnetzer GW, Reed JC. Modified VAD and PSC-833 in the treatment of resistant or relapsing chronic lymphocytic leukemia (E4996): a trial of the Eastern Cooperative Oncology Group. Leuk Res. 2004;28(8):813–9.
Carrion C, Madariaga MA, Domingo JC. In vitro cytotoxic study of immunoliposomal doxorubicin targeted to human CD34+ leukemic cells. Life Sci. 2004;75(3):313–28.
Sikic BI, Fisher GA, Lum BL, Halsey J, Beketic-Oreskovic L, Chen G. Modulation and prevention of multidrug resistance by inhibitors of P-glycoprotein. Cancer Chemother Pharmacol. 1997;40(7):S13–9.
Ueda K, Cardarelli C, Gottesman MM, Pastan I. Expression of a full-length cDNA for the human “MDR1”gene confers resistance to colchicine, doxorubicin, and vinblastine. Proc Natl Acad Sci USA. 1987;84(9):3004–8.
Gottesman MM, Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427.
Broxterman HJ, Sonneveld P, Feller N, Ossenkoppele GJ, Wahrer DC, Eekman CA, Schoester M, Lankelma J, Pinedo HM, Lowenberg B, Schuurhuis GJ, et al. Quality control of multidrug resistance assays in adult acute leukemia: correlation between assays for P-glycoprotein expression and activity. Blood. 1996;87(11):4809–16.
Hirose M, Hosoi E, Hamano S, Jalili A. Multidrug resistance in childhood hematologic malignancies. J Med Invest. 2003;50(3):126–35.
Arceci RJ. Clinical significance of P-glycoprotein in multidrug resistance malignancies. Blood. 1993; 81(9):2215–11.
Morjani H, Aouali N, Belhoussine R, Veldman RJ, Levada T, Manfait M. Elevation of glucosylceramide in multidrug-resistant cancer cells and accumulation in cytoplasmic droplets. Int Cancer. 2001;94(2):157–65.
Gouaze V, Yu JY, Bleicher RJ, Han TY, Liu YY, Wang H, Gottesman MM, Bitterman A, Giuliano AE, Cabot MC. Overexpression of glucosylceramide synthase and P-glycoprotein in cancer cells selected for resistance to natural product chemotherapy. Mol Cancer Ther. 2004;3(5):633–9.
Liu YY, Han TY, Giuliano AE, Hansen N, Myles C. Cabot Uncoupling ceramide glycosylation by transfection of glucosylceramide synthase antisense reverses adriamycin resistance. J Biol Chem. 2000;275(10):7138–43.
Ping X, Shen YF, Shi YP, Ge SM, Gu ZH, Wang J, Mu HJ, Zhang B, Qiao WZ, Xie KM, et al. Overexpression of glucosylceramide synthase in associated with multidrug resistance of leukemia cells. Leuk Res. 2008;32(3):475–80.
Ricci C, Onida F, Ghidoni R. Sphingolipid players in the leukemia arena. Biochim Biophys Acta. 2006;1758(12):2121–32.
Gouaze V, Liu YY, Carlton S, Prickett CS, Jing Y, Giuliano AE, Myles C, et al. Cabot glucosylceramide synthase blockade down-regulates P-glycoprotein and resensitizes multidrug-resistant breast cancer cells to anticancer drugs. Cancer Res. 2005;65(9):3861–7.
Liu Y, Xie KM, Yang GQ, Bai XM, Shi YP, Mu HJ, Qiao WZ, Bin Zhang B, Xie P. GCS induces multidrug resistance by regulating apoptosis-related genes in K562/AO2 cell line. Cancer Chemother Pharmacol. 2009;66(3):433–9.
Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976;455(1):152–62.
Huwiler A, Kolter T, Pfeilschifter J, Sandhoff K, et al. Physiology and pathophysiology of sphingolipid metabolism and signaling. Biochim Biophys Acta. 2000;1485(2-3):63–99.
Kohyama-koganaya A, Hirabayashi Y. Role of glucosylceramide synthase as negative regulator for ceramide. Tanpakushitsu Kakusan Koso. 2002;47(4 Suppl):470–5.
Gerrard G, Butters TD, Ganeshaguru K, Mehta AB. Glucosylceramide synthase inhibitors sensitise CLL cells to cytotoxic agents without reversing P-gp functional activity. Eur J Pharmacol. 2009;609(1–3):34–9.
De Rosa MF, Sillence D, Ackerley C, Lingwood C. Role of multiple drug resistance protein 1 in neutral but not acidic glycosphingolipid biosynthesis. J Biol Chem. 2004;279(9):7867–76.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Zhang, YY., Xie, KM., Yang, GQ. et al. The effect of glucosylceramide synthase on P-glycoprotein function in K562/AO2 leukemia drug-resistance cell line. Int J Hematol 93, 361–367 (2011). https://doi.org/10.1007/s12185-011-0798-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-011-0798-7