Abstract
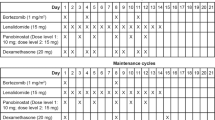
Bortezomib and doxorubicin have synergistic activity against myeloma cells in vitro. We underwent a dose finding study of bortezomib in combination with a fixed dose of doxorubicin and intermediate-dose dexamethasone (iPAD therapy) in patients with relapsed or refractory myeloma. Bortezomib was administered on days 1, 4, 8 and 11 at a dose of 1.0 and 1.3 mg/m2 in cohorts 1 and 2, respectively. Doxorubicin 9 mg/m2 was given by rapid intravenous infusion on days 1–4, and dexamethasone 20 mg on days 1–2, 4–5, 8–9 and 11–12. Treatment was repeated at a 3-week interval and the dose-limiting toxicity (DLT), defined as grade 4 hematological toxicity lasting more than 5 days and/or grade 3 or higher non-hematological toxicity, was evaluated. In cohort 1, 2 of 6 patients developed DLTs including grade 4 hyponatremia and grade 3 infection with appropriate neutrophil counts. No DLT was observed in the remaining 4 patients, indicating this dose was tolerable. In cohort 2, 3 of 5 patients developed DLTs including grade 4 thrombocytopenia lasting more than 5 days, grade 3 hepatic transaminase elevation and grade 3 ileus, indicating this dose was intolerable. It is concluded that bortezomib at the dose of 1.0 mg/m2 is recommended in combination with doxorubicin and intermediate-dose dexamethasone.
Similar content being viewed by others
References
Adams J. The proteasome: a suitable antineoplastic target. Nat Rev Cancer. 2004;4:349–60.
Hideshima T, Richardson P, Chauhan D, et al. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61:3071–6.
Jagannath S, Barlogie B, Berenson J, et al. A phase 2 study of two doses of bortezomib in relapsed or refractory myeloma. Br J Haematol. 2004;127:165–72.
Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–17.
Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–98.
Mitsiades N, Mitsiades CS, Richardson PG, et al. The proteasome inhibitor PS-341 potentiates sensitivity of multiple myeloma cells to conventional chemotherapeutic agents: therapeutic applications. Blood. 2003;101:2377–80.
Ma MH, Yang HH, Parker K, et al. The proteasome inhibitor PS-341 markedly enhances sensitivity of multiple myeloma tumor cells to chemotherapeutic agents. Clin Cancer Res. 2003;9:1136–44.
Oakervee HE, Popat R, Curry N, et al. PAD combination therapy (PS-341/bortezomib, doxorubicin and dexamethasone) for previously untreated patients with multiple myeloma. Br J Haematol. 2005;129:755–62.
Orlowski RZ, Nagler A, Sonneveld P, et al. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J Clin Oncol. 2007;25:3892–901.
Berenson JR, Yang HH, Sadler K, et al. Phase I/II trial assessing bortezomib and melphalan combination therapy for the treatment of patients with relapsed or refractory multiple myeloma. J Clin Oncol. 2006;24:937–44.
Mateos MV, Hernandez JM, Hernández MT, et al. Bortezomib plus melphalan and prednisone in elderly untreated patients with multiple myeloma: results of a multicenter phase 1/2 study. Blood. 2006;108:2165–72.
Kropff M, Bisping G, Schuck E, et al. Bortezomib in combination with intermediate-dose dexamethasone and continuous low-dose oral cyclophosphamide for relapsed multiple myeloma. Br J Haematol. 2007;138:330–7.
Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. N Engl J Med. 1996;335:91–7.
Pineda-Roman M, Zangari M, van Rhee F, et al. VTD combination therapy with bortezomib-thalidomide-dexamethasone is highly effective in advanced and refractory multiple myeloma. Leukemia. 2008;22:1419–27.
Popat R, Oakervee HE, Hallam S, et al. Bortezomib, doxorubicin and dexamethasone (PAD) front-line treatment of multiple myeloma: updated results after long-term follow-up. Br J Haematol. 2008;141:512–6.
Acknowledgments
This clinical study was registered in University hospital Medical Information Network (UMIN) (registration number; UMIN000001210), and was supported by the non-profit organization of Clinical Hematology/Oncology Treatment Study Group (CHOT-SG). There was no financial disclosure from any authors. We thank project managers Ms. Y. Ito and N. Gushima, and secretaries Ms. E. Kumakawa and N. Ikoma for valuable assistance in conducting the present study, and the medical staff of the following institutions for participating in this study; T. Toyota, Y. Ikari, H. Sasaki, K. Ishitsuka (Fukuoka University), T. Ochi, Y. Meguri (Okayama Medical Center), S. Murakami, M. Kugimiya (Kumamoto University), Y. Mori, Y. Abe, T. Miyamoto, T. Teshima (Kyushu University), N. Uike (Kyushu Cancer Center), K. Aoki (Kyushu Kosei-nenkin Hospital), D. Nakamura (Kagoshima University), M. Miki (Kanazawa Medical University), J. Suzumiya (Shimane University), J. Tsukada (University of Occupational and Environmental Health), T. Okamura, K. Yakushiji (Kumure University), T. Eto, K. Takase (Hamanomachi Hospital), Y. Imamura (St. Mary’s Hospital), F. Taguchi, Y. Tachikawa, Y. Nakashima (Iizuka Hospital), S. Makino (Toranomon Hospital), and S. Aoki (Niigata University).
Author information
Authors and Affiliations
Consortia
Corresponding author
About this article
Cite this article
Takamatsu, Y., Sunami, K., Hata, H. et al. A phase I study of bortezomib in combination with doxorubicin and intermediate-dose dexamethasone (iPAD therapy) for relapsed or refractory multiple myeloma. Int J Hematol 92, 503–509 (2010). https://doi.org/10.1007/s12185-010-0673-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-010-0673-y