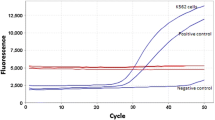
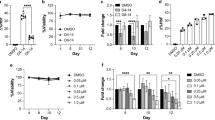
Abstract
The aim of the present study was to identify molecular analogs of angelicin (ANG) able to increase erythroid differentiation of K562 cells and expression of γ-globin genes in human erythroid precursor cells, with low effects on apoptosis. ANG-like molecules are well-known photosensitizers largely used for their antiproliferative activity in the treatment of different skin diseases (i.e., psoriasis, vitiligo, eczema, and mycosis fungoides). To verify the activity of these derivatives, we employed three experimental cell systems: (1) the human leukemic K562 cell line, (2) K562 cell clones stably transfected with a pCCL construct carrying green-EGFP under the γ-globin gene promoter, and (3) the two-phase liquid culture of human erythroid progenitors isolated from normal donors and β-thalassemia patients. The results of our study suggest that trimethyl ANG is a powerful inducer of erythroid differentiation, compared with known inducers, such as ANG, cytosine arabinoside, mithramycin, and cisplatin. These data could have practical relevance, because pharmacologically mediated regulation of human γ-globin gene expression, with the consequent induction of fetal hemoglobin, is considered a potential therapeutic approach in hematological disorders including β-thalassemia and sickle cell anemia.





Similar content being viewed by others
References
Rodgers GP, Rachmilewitz EA. Novel treatment options in the severe β-globin disorder. Br J Haematol. 1995;91:263–8.
Rochette J, Craig JE, Thein SL. Fetal hemoglobin levels in adults. Blood Rev. 1994;8:213–24.
Olivieri NF, Rees DC, Ginder DG, Thein SL, Waye JS, Chang L, et al. Elimination of transfusion through induction of fetal hemoglobin synthesis in Cooley’s anemia. Ann NY Acad Sci USA. 1998;850:100–9.
Swank RA, Stamatoyannopoulos G. Fetal gene reactivation. Curr Opin Genet Dev. 1998;8:366–70.
Steinberg MH, Lu ZH, Barton FB, Terrin ML, Charache S, Dover GJ. Fetal hemoglobin in sickle cell anemia: determinants of response to hydroxyurea: multicenter study of hydroxyurea. Blood. 1997;89:1078–88.
Fibach E, Prasanna P, Rodgers GP, Samid D. Enhanced fetal hemoglobin production by phenylacetate and 4-phenylbutyrate in erythroid precursors derived from normal donors and patients with sickle cell anemia and β-thalassemia. Blood. 1993;82:2203–9.
Rodgers GP, Dover GJ, Uyesaka N, Noguchi CT, Schechter AN, Nienhuis AW. Augmentation by erythropoietin of the fetal-hemoglobin response to hydroxyurea in sickle cells disease. N Engl J Med. 1993;328:73–80.
Perrine SP, Ginder GD, Faller DV, Dover GH, Ikuta T, Witkowska HE, et al. A short-term trial of butyrate to stimulate fetal-globin gene expression in the β-globin disorders. N Engl J Med. 1993;328:81–6.
Bianchi N, Osti F, Rutigliano C, Ginanni Corradini F, Borsetti E, Tomassetti M, et al. The DNA-binding drugs mithramycin and chromomycin are powerful inducers of erythroid differentiation of human K562 cells. Br J Haematol. 1999;104:258–63.
Bianchi N, Ongaro F, Chiarabelli C, Gualandi L, Mischiati C, Bergamini P, et al. Induction of erythroid differentiation of human K562 cells by cisplatin analogs. Biochem Pharmacol. 2000;60:31–40.
Bianchi N, Chiarabelli C, Borgatti M, Mischiati C, Fibach E, Gambari R. Accumulation of gamma-globin mRNA and induction of erythroid differentiation after treatment of human leukemia K562 cells with tallimustine. Br J Haematol. 2001;113:951–61.
Lampronti I, Bianchi N, Borgatti M, Fibach E, Prus E, Gambari R. Accumulation of gamma-globin mRNA in human erythroid cells treated with angelicin. Eur J Haematol. 2003;71:189–95.
Chilin A, Marzano C, Guiotto A, Manzini P, Baccichetti F, Carlassare F, et al. Synthesis and biological activity of (hydroxymethyl)- and (diethylaminomethyl)-benzopsoralens. J Med Chem. 1999;42:2936–45.
Bordin F, Marzano C, Baccichetti F, Carlassare F, Vedaldi D, Falcomer S, et al. Photobiological properties of 1′-thieno-4, 6, 4′-trimethylangelicin. Photochem Photobiol. 1998;68:157–63.
Conconi MT, Montesi F, Parnigotto PP. Antiproliferative activity and phototoxicity of some methyl derivatives of 5-methoxypsoralen and 5-methoxyangelicin. Pharmacol Toxicol. 1998;82:193–8.
Marzano C, Caffieri S, Fossa P, Bordin F. Activity of 3-carbethoxyangelicin photolysis products. J Photochem Photobiol B. 1997;38:189–95.
Bordin F, Dall’Acqua F, Guiotto A. Angelicins, angular analogs of psoralens: chemistry, photochemical, photobiological and phototherapeutic properties. Pharmacol Ther. 1991;52:331–63.
Guiotto A, Rodighiero P, Manzini P, Pastorini G, Bordin F, Baccichetti F, et al. 6-Methylangelicins: a new series of potential photochemotherapeutic agents for the treatment of psoriasis. J Med Chem. 1984;27:959–67.
Komura J, Ikehata H, Hosoi Y, Riggs AD, Ono T. Mapping psoralen cross-links at the nucleotide level in mammalian cells: suppression of cross-linking at transcription factor- or nucleosome-binding sites. Biochemistry. 2001;40:4096–105.
Mosti L, Lo Presti E, Menozzi G, Marzano C, Baccichetti F, Falcone G, et al. Synthesis of angelicin heteroanalogues: preliminary photobiological and pharmacological studies. Farmaco. 1998;53:602–10.
Lozzio BB, Lozzio CB. Properties of the K562 cell line derived from a patient with chronic myeloid leukemia. Int J Cancer. 1977;19(1):136.
Gambari R, Del Senno L, Barbieri R, Viola L, Tripodi M, Raschella G, et al. Human leukemia K-562 cells: induction of erythroid differentiation by 5-azacytidine. Cell Differ. 1984;14:87–97.
Fibach E, Manor D, Oppenheim A, Rachmilewitz EA. Proliferation and maturation of human erythroid progenitors in liquid medium. Blood. 1989;73:100–3.
Pope SH, Fibach E, Sun J, Chin K, Rodgers GP. Two phase liquid culture system models normal human adult erythropoiesis at the molecular level. Eur J Haematol. 2000;64:292–303.
Gambari R, Fibach E. Medicinal chemistry of fetal hemoglobin inducers for treatment of β-thalassemia. Curr Med Chem. 2007;14:199–212.
Zuccato C, Bianchi N, Borgatti M, Lampronti I, Massei F, Favre C, et al. Everolimus is a potent inducer of erythroid differentiation and gamma-globin gene expression in human erythroid cells. Acta Haematol. 2007;117:168–76.
Fibach E, Bianchi N, Borgatti M, Prus E, Gambari R. Mithramycin induces fetal hemoglobin production in normal and thalassemic human erythroid precursor cells. Blood. 2003;102:1276–81.
Fibach E, Bianchi N, Borgatti M, Zuccato C, Finotti A, Lampronti I, et al. Effects of rapamycin on accumulation of alpha-, beta- and gamma-globin mRNAs in erythroid precursor cells from β-thalassaemia patients. Eur J Haematol. 2006;77:437–41.
Quentmeier H, Zaborski M, Drexler HG. The human bladder carcinoma cell line 5637 constitutively secretes functional cytokines. Leuk Res. 1997;21:343–50.
Penolazzi L, Lambertini E, Borgatti M, Piva R, Cozzani M, Giovannini I, et al. Decoy oligodeoxynucleotides targeting NF-kappa B transcription factors: induction of apoptosis in human primary osteoclasts. Biochem Pharmacol. 2003;66:1189–98.
Piva R, Penolazzi L, Zennaro M, Bianchini E, Magri E, Borgatti M, et al. Induction of apoptosis of osteoclasts by targeting transcription factors with decoy molecules. Ann N Y Acad Sci. 2006;1091:509–16. Review.
Lampronti I, Martello D, Bianchi N, Borgatti M, Lambertini E, Piva R, et al. In vitro antiproliferative effects on human tumor cell lines of extracts from the Bangladeshi medicinal plant Aegle marmelos Correa. Phytomedicine. 2003;10:300–8.
Bianchi N, Zuccato C, Lampronti I, Borgatti M, Gambari R. Fetal hemoglobin inducers from the natural world: a novel approach for identification of drugs for the treatment of β-thalassemia and sickle-cell anemia. Evid Based Complement Alternat Med. 2009;6:141–51.
Parrish JA. Phototherapy and photochemotherapy of skin diseases. J Invest Dermatol. 1981;77:167–71.
Cristofolini M, Recchia G, Boi S, Piscioli F, Bordin F, Baccichetti F, et al. 6-Methylangelicins: new monofunctional photochemotherapeutic agents for psoriasis. Br J Dermatol. 1990;122:513–24.
Acknowledgment
R.G. is granted by AIRC, Fondazione Cariparo (Cassa di Risparmio di Padova e Rovigo), Cofin-2005, by STAMINA Project (University of Ferrara), by UE ITHANET Project and by Telethon Project (GGP07257). This research was also supported by Regione Emilia–Romagna (Spinner Project) and by Associazione Veneta per la Lotta alla Talassemia, Rovigo.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Lampronti, I., Bianchi, N., Zuccato, C. et al. Increase in γ-globin mRNA content in human erythroid cells treated with angelicin analogs. Int J Hematol 90, 318–327 (2009). https://doi.org/10.1007/s12185-009-0422-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-009-0422-2