Abstract
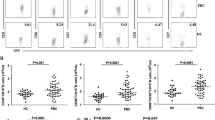
To investigate the association between hepatitis C virus (HCV) and B cell proliferation, we searched for the clonal B cells by flow cytometric analysis of the surface immunoglobulin kappa (κ):lambda (λ) light chain ratios of the circulating B (CD19+) cells in 240 HCV-positive patients and 150 negative controls with liver diseases. Clonal B cells with light chain restriction (κ:λ ratio >3:1 or <1:2) were analyzed for CD5 expression and the presence of monoclonal immunoglobulin heavy-chain (IGH) gene rearrangements and the t(14;18) chromosomal translocation. Clonal B cells were detected in 7 cases with HCV (2.9%), but was never detected in the controls (p < 0.05). Of the 7 cases, all had monoclonal IGH gene rearrangements and one had the t(14;18) chromosomal translocation. These HCV-related clonal B cells are not uniform in the intensity of CD5 expression and showed no increase in the frequencies of CD5+ population compared with non-clonal B cells. No “chronic lymphocytic leukemia-phenotype” cells were found. The loss of clonality was observed in 2 cases treated with interferon and in one case treated with splenectomy. The longitudinal study is required to determine whether these circulating clonal B cells progress to lymphoproliferative disorders in future or not.





Similar content being viewed by others
References
Blackard JT, Kemmer N, Sherman KE. Extrahepatic replication of HCV: insights into clinical manifestations and biological consequences. Hepatology. 2006;44:15–22. doi:10.1002/hep.21283.
Morsica G, Tambussi G, Sitia G, Novati R, Lazzarin A, Lopalco L, et al. Replication of hepatitis C virus in B lymphocytes (CD19+). Blood. 1999;94:1138–9.
Ohsawa M, Shingu N, Miwa H, Yoshihara H, Kubo M, Tsukuma H, et al. Risk of non-Hodgkin’s lymphoma in patients with hepatitis C virus infection. Int J Cancer. 1999;80:237–9. doi:10.1002/(SICI)1097-0215(19990118)80:2<237::AID-IJC12>3.0.CO;2-I.
Giordano TP, Henderson L, Landgren O, Chiao EY, Kramer JR, El-Serag H, et al. Risk of non-Hodgkin lymphoma and lymphoproliferative precursor diseases in US veterans with hepatitis C virus. JAMA. 2007;297:2010–7. doi:10.1001/jama.297.18.2010.
Ferri C, Caracciolo F, Zignego AL, Civita LL, Monti M, Longombardo G, et al. Hepatitis C virus infection in patients with non-Hodgkin’s lymphoma. Br J Haematol. 1994;88:392–4. doi:10.1111/j.1365-2141.1994.tb05036.x.
Mele A, Pulsoni A, Bianco E, Musto P, Szklo A, Sanpaolo MG, et al. Hepatitis C virus and B-cell non-Hodgkin lymphomas: an Italian multicenter case-control study. Blood. 2003;102:996–9. doi:10.1182/blood-2002-10-3230.
Hermine O, Lefrère F, Bronowicki JP, Mariette X, Jondeau K, Eclache-Saudreau V, et al. Regression of splenic lymphoma with villous lymphocytes after treatment of hepatitis C virus infection. N Engl J Med. 2002;347:89–94. doi:10.1056/NEJMoa013376.
Vallisa D, Bernuzzi P, Arcaini L, Sacchi S, Callea V, Marasca R, et al. Role of anti-hepatitis C virus (HCV) treatment in HCV-related, low-grade, B-cell, non-Hodgkin’s lymphoma: a multicenter Italian experience. J Clin Oncol. 2005;23:468–73. doi:10.1200/JCO.2005.06.008.
Ivanovski M, Silvestri F, Pozzato G, Anand S, Mazzaro C, Burrone OR, et al. Somatic hypermutation, clonal diversity, and preferential expression of the VH 51p1/VL kv325 immunoglobulin gene combination in hepatitis C virus-associated immunocytomas. Blood. 1998;91:2433–42.
De Re V, De Vita S, Marzotto A, Gloghini A, Pivetta B, Gasparotto D, et al. Pre-malignant and malignant lymphoproliferations in an HCV-infected type II mixed cryoglobulinemic patient are sequential phases of an antigen-driven pathological process. Int J Cancer. 2000;87:211–6. doi:10.1002/1097-0215(20000715)87:2<211::AID-IJC9>3.0.CO;2-8.
Quinn ER, Chan CH, Hadlock KG, Foung SK, Flint M, Levy S. The B-cell receptor of a hepatitis C virus (HCV)-associated non-Hodgkin lymphoma binds the viral E2 envelope protein, implicating HCV in lymphomagenesis. Blood. 2001;98:3745–9. doi:10.1182/blood.V98.13.3745.
Machida K, Cheng KT, Pavio N, Sung VM, Lai MM. Hepatitis C virus E2-CD81 interaction induces hypermutation of the immunoglobulin gene in B cells. J Virol. 2005;79:8079–89. doi:10.1128/JVI.79.13.8079-8089.2005.
Zuckerman E, Zuckerman T, Sahar D, Streichman S, Attias D, Sabo E, et al. Bcl-2 and immunoglobulin gene rearrangement in patients with hepatitis C virus infection. Br J Haematol. 2001;112:364–9. doi:10.1046/j.1365-2141.2001.02573.x.
Vallat L, Benhamou Y, Gutierrez M, Ghillani P, Hercher C, Thibault V, et al. Clonal B cell populations in the blood and liver of patients with chronic hepatitis C virus infection. Arthritis Rheum. 2004;50:3668–78. doi:10.1002/art.20594.
Tatu C, Ye J, Arnold LW, Clarke SH. Selection at multiple checkpoints focuses V(H)12 B cell differentiation toward single B-1 cell specificity. J Exp Med. 1999;190:903–14. doi:10.1084/jem.190.7.903.
Hardy RR, Wei CJ, Hayakawa K. Selection during development of VH11+ B cells: a model for natural autoantibody-producing CD5+ B cells. Immunol Rev. 2004;197:60–74. doi:10.1111/j.0105-2896.2004.0100.x.
Curry MP, Golden-Mason L, Nolan N, Parfrey NA, Hegarty JE, O’Farrelly C. Expansion of peripheral blood CD5+ B cells is associated with mild disease in chronic hepatitis C virus infection. J Hepatol. 2000;32:121–5. doi:10.1016/S0168-8278(00)80198-1.
Zuckerman E, Kessel A, Slobodin G, Sabo E, Yeshurun D, Toubi E. Antiviral treatment down-regulates peripheral B-cell CD81 expression and CD5 expansion in chronic hepatitis C virus infection. J Virol. 2003;77:10432–6. doi:10.1128/JVI.77.19.10432-10436.2003.
Cacoub P, Bourlière M, Hausfater P, Charlotte F, Khiri H, Toci S, et al. Lower expression of CD81 B-cell receptor in lymphoproliferative diseases associated with hepatitis C virus infection. J Viral Hepat. 2003;10:10–5. doi:10.1046/j.1365-2893.2003.00380.x.
Ni J, Hembrador E, Di Bisceglie AM, Jacobson IM, Talal AH, Butera D, et al. Accumulation of B lymphocytes with a naive, resting phenotype in a subset of hepatitis C patients. J Immunol. 2003;170:3429–39.
Pasquali JL, Waltzinger C, Kuntz JL, Knapp AM, Levallois H. The majority of peripheral blood monoclonal IgM secreting cells are CD5 negative in three patients with mixed cryoglobulinemia. Blood. 1991;77:1761–5.
Crouzier R, Martin T, Pasquali JL. Monoclonal IgM rheumatoid factor secreted by CD5-negative B cells during mixed cryoglobulinemia. Evidence for somatic mutations and intraclonal diversity of the expressed VH region gene. J Immunol. 1995;154:413–21.
Tamura K, Sawada H, Izumi Y, Fukuda T, Utsunomiya A, Ikeda S, et al. Chronic lymphocytic leukemia (CLL) is rare, but the proportion of T-CLL is high in Japan. Eur J Haematol. 2001;67:152–7. doi:10.1034/j.1600-0609.2001.5790514.x.
Segal GH, Edinger MG, Owen M, McNealis M, Lopez P, Perkins A, et al. Concomitant delineation of surface Ig, B-cell differentiation antigens, and HLADR on lymphoid proliferations using three-color immunocytometry. Cytometry. 1991;12:350–9. doi:10.1002/cyto.990120410.
Kawano-Yamamoto C, Muroi K, Izumi T, Saito K, Ozawa K. Two-color flow cytometry with a CD19 gate for the evaluation of bone marrow involvement of B-cell lymphoma. Leuk Lymphoma. 2002;43:2133–7. doi:10.1080/1042819021000033051.
Ramasamy I, Brisco M, Morley A. Improved PCR method for detecting monoclonal immunoglobulin heavy chain rearrangement in B cell neoplasms. J Clin Pathol. 1992;45:770–5. doi:10.1136/jcp.45.9.770.
Seto M, Osada H, Ueda R, Ito C, Iwaki O, Oyama A, et al. Bcl-2 translocation in Japanese B cell lymphoma: novel bcl-2 translocation with immunoglobulin heavy chain diversity segment. Jpn J Cancer Res. 1991;82:65–71.
Ngan BY, Nourse J, Cleary ML. Detection of chromosomal translocation t(14;18) within the minor cluster region of bcl-2 by polymerase chain reaction and direct genomic sequencing of the enzymatically amplified DNA in follicular lymphomas. Blood. 1989;73:1759–62.
Kruetzmann S, Rosado MM, Weber H, Germing U, Tournilhac O, Peter HH, et al. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J Exp Med. 2003;197:939–45. doi:10.1084/jem.20022020.
Di Sabatino A, Rosado MM, Ciccocioppo R, Cazzola P, Morera R, Corazza GR, et al. Depletion of immunoglobulin M memory B cells is associated with splenic hypofunction in inflammatory bowel disease. Am J Gastroenterol. 2005;100:1788–95. doi:10.1111/j.1572-0241.2005.41939.x.
Carbonari M, Caprini E, Tedesco T, Mazzetta F, Tocco V, Casato M, et al. Hepatitis C virus drives the unconstrained monoclonal expansion of VH1–69-expressing memory B cells in type II cryoglobulinemia: a model of infection-driven lymphomagenesis. J Immunol. 2005;174:6532–9.
Sansonno D, Tucci FA, De Re V, Lauletta G, Montrone M, Libra M, et al. HCV-associated B cell clonalities in the liver do not carry the t(14;18) chromosomal translocation. Hepatology. 2005;42:1019–27. doi:10.1002/hep.20887.
Libra M, Gloghini A, Malaponte G, Gangemi P, De Re V, Cacopardo B, et al. Association of t(14;18) translocation with HCV infection in gastrointestinal MALT lymphomas. J Hepatol. 2008;49:170–4. doi:10.1016/j.jhep.2008.03.031.
Acknowledgments
This work was supported by Research on Hepatitis, Health and Labour Sciences Research Grants from the Ministry of Health, Labour, and Welfare of Japan. K.O., T.O., M.S., T.U., T.K., S.M., Y.T., E.O., R.I., R.S., M.H., K.O., K.Y., T.K., and K.Y participated in devising the study concept; M.S. and T.K. provided patient referrals; K.O. performed and T.U. assisted experiments; K.O. and T.O. designed the research and wrote the paper.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Ohtsubo, K., Sata, M., Kawaguchi, T. et al. Characterization of the light chain-restricted clonal B cells in peripheral blood of HCV-positive patients. Int J Hematol 89, 452–459 (2009). https://doi.org/10.1007/s12185-009-0301-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-009-0301-x