Abstract
An experimental study was performed to investigate the impact of low salinity water on wettability alteration in carbonate core samples from southern Iranian reservoirs by spontaneous imbibition. In this paper, the effect of temperature, salinity, permeability and connate water were investigated by comparing the produced hydrocarbon curves. Contact angle measurements were taken to confirm the alteration of surface wettability of porous media. Oil recovery was enhanced by increasing the dilution ratio of sea water, and there existed an optimum dilution ratio at which the highest oil recovery was achieved. In addition, temperature had a very significant impact on oil recovery from carbonate rocks. Furthermore, oil recovery from a spontaneous imbibition process was directly proportional to the permeability of the core samples. The presence of connate water saturation inside the porous media facilitated oil production significantly. Also, the oil recovery from porous media was highly dependent on ion repulsion/attraction activity of the rock surface which directly impacts on the wettability conditions. Finally, the highest ion attraction percentage was measured for sodium while there was no significant change in pH for all experiments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Currently, increasing oil production through enhanced oil recovery (EOR) techniques is one of the important subjects that many researchers are studying in carbonate and sandstone reservoirs (Austad et al. 2010; Lager et al. 2008a; Mahani et al. 2015; Shehata and Nasr El-Din 2015; Tang and Morrow 1999). More than 60% of world’s oil is trapped in carbonate reservoirs. These reservoirs which play an important role in world oil production are very complex and contain fractures with different sizes and lengths ranging from small fissures to kilometer-wide features. The communication between matrix and fractures via gravity and capillary forces is the main mechanism controlling the production from fractured reservoirs (Firoozabadi 2000). However, a considerable amount of oil remains in the matrix block. The main reason for high remaining oil saturation in the carbonate reservoirs is that the rock is oil-wet (Al-Hadhrami and Blunt 2000; Hirasaki and Zhang 2004; Standnes and Austad 2000). Much of the oil in the carbonate reservoirs is trapped in the rock matrix (Firoozabadi 2000).
There exist several methods to recover the trapped oil from the matrix medium (Green and Willhite 1998; Shaddel and Tabatabae-Nejad 2015; Shaker Shiran and Skauge 2013; Simjoo et al. 2015) in which wettability alteration by low salinity water is one of the most effective techniques (Navratil 2012; Shehata and Nasr El-Din 2015; Zahid et al. 2012). Much experimental work has been conducted to investigate the impact of low salinity water on wettability alteration, mainly focusing on spontaneous imbibition (Shehata and Nasr El-Din 2015; Wickramathilaka et al. 2011) and core flooding experiments (Nasralla et al. 2011; Morrow and Buckley 2011; Rivet et al. 2010; Zahid et al. 2012). It should be noted that an immersed core sample represents a matrix medium while the brine-filled gap between the core and glass acts as a fracture in the Amott test. Experimental results indicate that low salinity water injected into the cell could alter the wettability of the rock from oil-wet to water-wet (Morrow and Buckley 2011; Patil et al. 2008; Zahid et al. 2012). Shaddel and Tabatabae-Nejad (2015) investigated the impact of low salinity water on oil recovery performance of low-permeability core samples from a sandstone reservoir. In their study, up to 5% oil recovery enhancement was obtained by low salinity water injection after high salinity injection scenarios. In addition, higher recovery was observed at higher dilution ratio of formation water (100 times diluted). Furthermore, electrical double layer expansion and multi-component ion exchange were determined as recovery mechanisms without any pH impact on oil recovery (Shaddel and Tabatabae-Nejad 2015).
Shaker Shiran and Skauge (2013) performed an experimental study on sandstone core to compare the effect of salinity on recovery curves. A significant oil recovery enhancement was achieved with low salinity water injection which shows good agreement with findings of other researchers. In addition, they reported that the mixed wettability situation is more effective than water-wet conditions in terms of oil production. As a conclusion based on many studies in this area, low salinity water improves oil recovery in both spontaneous imbibition and core flooding tests. Furthermore, oil recovery would be increased by increasing the dilution ratio (Kulathu et al. 2013; McGuire et al. 2005; Patil et al. 2008; Shaddel and Tabatabae-Nejad 2015; Shaddel et al. 2014; Shaker Shiran and Skauge 2013; Torrijos et al. 2016; Wickramathilaka et al. 2011). It should be noted that no impact on oil recovery was observed in experiments with zero salinity content. Several imbibition experiments were conducted by Simjoo et al. (2015) on low-permeability (less than 10 mD) calcite cores with distilled water at 80 °C. In this experiment, crude oil used had an oil gravity of 17.9° API and contained 11.32% asphaltene. No oil production was obtained during the imbibition test (Simjoo et al. 2015). Therefore, there exists an optimum dilution ratio at which the highest oil recovery may be obtained.
Low salinity water injection experiments were performed by diluting saline water taken from the formation (Patil et al. 2008; Shaddel and Tabatabae-Nejad 2015), sea water (Shaker Shiran and Skauge 2012; Wickramathilaka et al. 2011; Zahid et al. 2012) or an artificial solution with desired salt concentrations. It is costly to provide formation water for the experiments while sea water is cheaper and more appropriate to conduct injection and imbibition tests.
Mahani et al. (2015) focused on mechanisms of wettability alteration by low salinity flooding in carbonate rocks through measuring contact angles and ζ-potentials. They suggested a combination of mechanisms including surface charge change and mineral dissolution, the former being the most effective and the latter having a positive but insignificant effect. Experimental results were in favor of their proposals. Contact angle results revealed that a decrease in water salinity could significantly make the rock surface more water-wet, and ζ-potential measurements demonstrated that reducing the salinity to a certain level would cause a less positive rock surface which is responsible for less attraction between rock and oil (Mahani et al. 2015).
Brady and Thyne (2016) investigated a model quantifying electrostatic adhesion between oil and carbonate rocks which accurately predicts oil recovery. This research has successfully indicated the positive surface charges of carbonate rocks at pH > 6, the effect of potential-determining ions on the surface charge and the effect of connate water on oil-rock adhesion (Brady and Thyne 2016).
Shariatpanahi et al. (2016) used dolomite outcrops to perform spontaneous imbibition tests and concluded that sea water did not act as a strong wettability modifier for dolomites at 70 °C, but using 10 times diluted sea water could increase the oil recovery by 10%–15% of original oil in place (OOIP) compared with the results from imbibition results with sea water (Shariatpanahi et al. 2016).
Sari et al. (2017) observed a linear relation between contact angle and ζ-parameter (a combination of rock-brine and oil-brine ζ-potential), stating that ζ-potential is a more reliable means of wettability alteration prediction rather than brine salinity because different salinities of different ions may result in the same oil recovery. However, what seems to be the main cause of wettability alteration is a shift in ζ-potential (Sari et al. 2017).
In addition to proving the effectiveness of low salinity water injection, several researchers investigated the important parameters affecting this EOR technique. Temperature is one of the effective parameters affecting oil recovery in low salinity water injection. Zhang et al. (2007) investigated the enhancement of oil recovery by temperature increase in low-permeability (less than 5 mD) chalk outcrop samples with spontaneous imbibition experiments. More oil was produced by increasing the temperature compared with low temperature experiments. At elevated temperatures, ion exchange affinity between imbibing water and the rock surface increases and consequently, wettability alteration occurs easily in the medium. Therefore, oil recovery increases at elevated temperatures (Zhang et al. 2007). When temperature increased a significant increase in oil recovery was observed in spontaneous imbibition tests performed on a sandstone core sample of permeability of about 200 mD (Shehata and Nasr El-Din 2015). The production time decreased at higher temperatures for obtaining a certain recovery percentage. Fast recovery at initial experimental time is contributed to volumetric expansion of oil. In addition, the oil to water viscosity ratio decreases with temperature rise, and consequently, water penetrates into the porous media with less resistance. Therefore, wettability alteration occurs with more speed at higher temperatures compared with lower temperature conditions (Shehata and Nasr El-Din 2015). Furthermore, Schembre et al. (2006) found that temperature changed the recovery factor from 12% to 43% in spontaneous imbibition tests. In this study, flooding was performed after imbibition tests on the same core samples at different temperatures. Experimental results confirmed the important role of temperature in recovering oil from core samples and a significant increase in wettability indices at higher temperatures. In addition, the impact of initial wettability conditions on oil recovery has been investigated (Schembre et al. 2006).
The core flooding method has been applied in most studies of low salinity water injection in which pressure exists as an effective parameter in the final obtained recovery. To eliminate the pressure impact, a spontaneous imbibition test is used as a replacement technique to evaluate the effectiveness of low salinity water injection. In general, very little information is available on the low salinity spontaneous imbibition in carbonate core samples since most of the published data are on sandstone samples. So, more experimental data and mechanistic studies are needed to investigate different aspects of low salinity water injection in carbonate reservoirs. The purpose of this study is to examine the importance of some parameters affecting the oil recovery by low salinity water injection in spontaneous imbibition tests. To this end, a series of tests were conducted in Amott cells to measure oil recovery by changing the desired parameters. The effect of sea water dilution ratio was investigated by measuring the recovered oil from Amott cells. In addition, mechanisms such as pH (McGuire et al. 2005), multi-component ion exchange (Lager et al. 2008b) and salting in (Rezaeidoust et al. 2009) were evaluated throughout this study by measuring the experimental data before and after each experiment. Furthermore, the effects of temperature, core permeability and connate water were also investigated by analyzing the recovery curves of designed experiments.
2 Materials and methods
2.1 Rock properties
To prepare core samples for imbibition experiments, standard core plugs were cut from a whole core which was taken from a carbonate reservoir located in the south of Iran. No signs of microfractures or vugs were detected in these core plugs. The carbonate core plugs had a diameter of 3.8 cm and length of 4–5 cm, approximately. Table 1 shows the physical properties of these core plugs in detail. In general, the core porosity ranged from 17.25% to 20.05%. An outcrop sample (Core 4) which had much higher permeability than others was used to investigate the permeability effect on oil recovery in the spontaneous imbibition test. Table 2 shows the X-ray diffraction (XRD) data of an identical core sample to those used in the experiments. Obviously, the samples were carbonate core plugs, with 94% calcite and 6% dolomite.
2.2 Crude oil
Carbonate core plugs were saturated with crude oil (dead oil) taken from a carbonate oil reservoir. The measured API gravity and viscosity of the oil were 32.84° API and 8.54 cP at 20 °C, respectively. Other properties of the crude oil are demonstrated in Table 3. It should be noted that the presence of asphaltene and wax in crude oil would have a significant effect on wettability conditions of porous media.
2.3 Brine
The brine used for imbibition tests was sampled from the Persian Gulf and was diluted at different ratios. Table 4 shows the cation composition of the sea water and diluted brines in detail. As can be observed, the focus of this study is on cations since anions are adsorbed on positively charged carbonate surfaces while oil is desorbed. Experiments were conducted using 5, 10, 20 and 40 times diluted sea water (named 5-tdsw, 10-tdsw, 20-tdsw, 40-tdsw) to investigate the optimum dilution ratio which yields the highest oil recovery during imbibition tests.
2.4 Core plug preparation
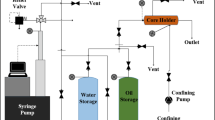
Oil, water and other materials in the core plugs were removed using a soxhlet apparatus with toluene and methanol as extracting solvent. Toluene (99.99%, Isfahan Petrochemical Company, Isfahan, Iran) and methanol (99.99%, Shiraz Petrochemical Company, Shiraz, Iran) were used successively to wash and clean core plugs before experiments. After this step, the core plugs were placed in a programmable oven at 110 °C for 6 h to evaporate any remaining solvents. In the next stage, the dried core plugs were saturated with dead oil in a vacuum desiccator at room temperature. Figure 1 shows a schematic of a system designed to saturate the core plugs. Air was removed from the pores of core plugs by a vacuum pump connected to the desiccator for 12 h, and then, the line of vacuum pump was closed. At the same time, an oil supply line was opened and oil droplets fell on the evacuated core plugs and then the oil penetrated into the porous medium. The oil line was closed when core plugs were fully submerged. Core plugs for Tests 1–8 were prepared using the above procedure; however, for Test 9 the core plug was initially saturated with water instead of oil and then flooded with oil to obtain connate water saturation.
A core flooding system (Fig. 2) was used to flood the core plugs with the same oil to complete the saturation process. Also, this apparatus was used to obtain the connate water saturation. To this end, the experimental procedures are as follows: The core plug was (1) evacuated and then saturated with water as depicted in Fig. 1, (2) placed inside the core holder as shown in Fig. 2 to be ready for flooding, (3) flooded with water (only in Test 9 in which connate water saturation was desired) and (4) flooded with crude oil with an injection rate of 0.1 mL/min at room temperature. In this step, absolute permeability was also measured. Several pore volumes of oil were injected into the core plug to ensure that all producible water was purged out of the core plug. By material balance, trapped water inside the core was calculated. Tests 1–8 did not include flooding with water (step 3).
2.5 Wettability measurement
It is very important to stabilize a desired wettability condition on core samples. During the core preparation, saturation and experiments, different wettability conditions may occur on the rock surface. The sessile drop method was used to measure the wettability alteration on core slabs taken from core plugs. Figure 3 shows a schematic of the sessile drop technique for contact angle measurement. A selected slab was placed in a container which was surrounded by brine and then an oil drop was injected by a syringe from the bottom of the container which was positioned a few millimeters away from the slab surface. A camera was placed to take a photograph during the oil droplet spreading process. The angle between the oil droplet and the rock surface was determined by photograph analysis. The contact angle reflects the wettability of the rock surface. The contact angle is between 0° and 180°. If the oil droplets completely spread out on the core slab, then 180° is the reading value for contact angle while 0° shows completely water-wet surface.
All slabs were treated with the same processes that the core plugs had been experienced during the imbibition experiments. Photographs were taken during all of these processes to measure the contact angle in each stage. Figure 4 shows the used contact angle measurement setup for this study.
Generally, all core plugs were water-wet after cleaning with toluene and methanol and then the surface had been changed to mild oil-wet during saturation with oil. In the aging process, all oil-saturated core plugs were immersed in crude oil for 14 days at 80 °C. In this step, the core slab photograph analysis showed oil-wet behavior for selected samples. An example of contact angle measurement and photograph analysis is presented in the results section of this paper.
2.6 Amott test
Figure 5a depicts a schematic of a matrix-fracture system in which spontaneous imbibition between matrix and fractures was simulated by an Amott cell (Fig. 5b). The matrix section is represented by a cylindrical core plug, and imbibing water represents the fracture section (Hatiboglu and Babadagli 2004). In the absence of connate water saturation, an oil-saturated core plug was placed in a typical Amott cell filled with brine as shown in Fig. 5b. The Amott cell was sealed precisely to avoid any leak from the glass container and placed in the programmable oven at the desired temperature. As soon as the setup was completed, the imbibition time was recorded. Oil was expelled from the core plug by spontaneous imbibition until a capillary equilibrium was reached. In the process of spontaneous imbibition, low salinity water penetrated into the porous media and altered the wettability of core surface by desorption of ions which were attached to the core surface. In this condition, the negative capillary pressure is changed to a positive value and consequently displaced oil out of the core plug. The oil expelled from the core plug was measured by reading the graduation on top of the Amott cell against time. The cumulative oil recovery versus time was displayed as the percentages of initial oil in-place (IOIP).
All Amott tests were performed on low-permeability core plugs at an elevated temperature of 75 °C except the tests which were designed to investigate the effect of temperature. In order to investigate the effect of temperature on oil recovery, two additional tests were conducted at 35 and 55 °C, respectively. In addition, different dilution ratios of low salinity water were prepared to investigate the effect of cation concentrations on oil recovery; also one Amott test was conducted in the presence of connate water to investigate the effect of connate water on oil recovery. In all tests, cation concentrations and pH values of water were measured before and after each test using an atomic absorption spectrometer and a pH meter, respectively, to evaluate the changes in cation content and pH. Furthermore, a high-permeability core plug was used to study the effect of rock permeability on oil recovery. Table 5 shows experimental specifications used in imbibition tests.
3 Results and discussion
The results of all conducted experiments are presented in this section to evaluate the impact of concentration, temperature, connate water and permeability on the oil recovery by low salinity water imbibition for carbonate core plugs. Before starting to present the experimental results of low salinity water, as an example, the photograph analysis of wettability alteration for one experiment is demonstrated. All photographs taken during the wettability measurements were analyzed by Digimizer software, version 4.1.1.0. Figure 6 shows contact angles of oil droplets on core slabs under different wettability conditions in preparation steps. It can be observed that the core slab was water-wet after cleaning with methanol and toluene (Fig. 6a, θ = 26.39°).
Photographs of core slabs treated with the same processes that core plugs have been experienced. a Core slab after cleaning with ethanol and toluene, θ = 26.39°. b Oil-saturated core slab after aging at 75 °C for 2 weeks, θ = 156.03°. c Oil-saturated core slab in contact with distilled water for 51 days at 75 °C, θ = 146.54°. d Core slab in contact with 20-tdsw for 51 days at 75 °C, θ = 40.23°
As it can be observed in Fig. 6b, the core slab became strongly oil-wet after aging treatment (Fig. 6b, θ = 156.03°) restoring the actual wettability under reservoir conditions.
Contact angles of crude oil on the core slabs after immersing in distilled water, 40-tdsw, 20-tdsw and 10-tdsw at 75 °C for different times are shown in Fig. 7. Contact angle experiments confirmed the wettability alteration by low salinity water. Minor fluctuations in the curves might be caused by measurement hysteresis due to slab surface roughness.
As an example, the results of two experiments were compared to demonstrate the effectiveness of ion presence in imbibing water for wettability alteration. It can be seen from Fig. 7 that no significant change in wettability was observed when the core slab was treated with distilled water, while a considerable wettability alteration was observed for low salinity water experiments; after contact with 20-tdsw for 62 days (1488 h) the contact angle decreased to 30o. Figure 6c, d shows contact angles of oil droplet on core slabs for the above-mentioned experiments. It can be observed that in 51 days of experiments with distilled water a meager change in wettability was detected on the core slab (Fig. 6c, θ = 146.5°). The higher contact angle achieved by distilled water could be explained by the fact that distilled water has no ion strength and therefore cannot feature any low salinity effect as discussed in the mechanisms. The presence of salinity in the medium could alter the wettability conditions toward more water-wet which is recognized by a significant reduction of contact angle. On the other hand, low salinity water altered the wetness of the surface to a completely different behavior (Fig. 6d, θ = 40.23°). It can be concluded that low salinity water has a great influence on surface properties of porous rock by altering the wetness of the medium with an ionic adsorption/desorption mechanism. In this regard, enhanced oil recovery by low salinity water can be considered as a potential technique to increase oil production from carbonate reservoirs. Regarding the different behavior of 10-tdsw, 20-tdsw and 40-tdsw in Figs. 7 and 8, it should be noted that two mechanisms affect the behavior of contact angle curves: mineral dissolution (dissolution of Ca from rock into the brine which requires low salinity water) and ion exchange (which is determined by ionic strength of the medium). The overall contact angle change is a result of these two forces acting in the medium. 10-tdsw is a rather high salinity brine and mostly benefits from the “ion exchange” mechanism, while 40-tdsw benefits from mainly the “mineral dissolution” process. 20-tdsw has both ion exchange capability and mineral dissolution driving force. The overall force balance in the start of the experiment is lower than 10-tdsw and 40-tdsw. As can be observed in the curves, both contact angle curves for 10-tdsw and 40-tdsw have a sharp drop at first, but have plateau at the end, showing loss of driving force. On the other hand, 20-tdsw keeps the driving force even after 800 h and passes the other two curves.
3.1 Water salinity effect
Five tests were conducted to investigate the effect of brine concentration on oil recovery from carbonate core plugs by spontaneous imbibition. As seen from Table 5, all tests were performed at an elevated temperature of 75 °C. Figure 8 shows the oil recovery (as fraction of IOIP) versus time for core plugs (1–3 and 5) using brines with different salinities. The first test was performed with distilled water as a base to observe the oil production from the carbonate core plug in the absence of any salinity. As expected, the oil production was meager when there was no salinity in the surrounding water. This observation was in good agreement with the findings of other researchers (Simjoo et al. 2015). In addition, experimental results of Tests 2–5 (40-tdsw, 20-tdsw, 10-tdsw and 5-tdsw) confirmed the effectiveness of ions in the imbibition water by producing a considerable amount of oil from core plugs.
As can be observed, lowering the salinity of imbibing water would improve oil recovery of carbonate core plugs. The effect of low salinity water in porous media on enhanced oil recovery is explained by the following mechanisms. A reduction in water salinity causes a thicker water layer to form on the mineral surface compared to high salinity water. In this condition, expansion of the water layer creates a higher opportunity for ions to exchange, and consequently, oil is removed from the rock surface with higher probability (Lee et al. 2010).
Expansion of the electrical double layer is another mechanism which is considered an explanation for recovery enhancement. In this mechanism, oil adsorbed on the rock surface is displaced by low salinity water via swelling of the electrical double layer. In low salinity water, the ionic strength decreases and consequently the thickness of the electrical double layer increases. Therefore, ion exchange occurs with higher opportunity which results in desorption of oil from the rock surface (Lager et al. 2007). However, there exists an optimum dilution ratio at which the highest oil recovery was obtained. In other words, reducing the salinity of water from a certain value is found to have an inverse effect on the oil production. In this study, it is observed that the highest ultimate oil recovery, 13.9% IOIP, was obtained at a dilution ratio of 20. So, this dilution ratio was selected as the optimum dilution ratio and the next series of tests were conducted with 20-tdsw.
It should be noted that in the process of oil recovery, imbibition time plays an important role. The highest ultimate oil recovery, 13.9% obtained at the end of Amott tests in Test 3 with 20-tdsw was achieved in a long-term spontaneous imbibition process (960 h). However, if the spontaneous imbibition time was short (i.e., 200 h), the highest oil recovery, 11.5% IOIP, was obtained at a dilution ratio of 10 (Test 4).
Before and after Amott tests, the ion concentrations in the imbibing water were measured to investigate multi-component ion exchange mechanisms. As previously discussed in contact angle measurements, the wettability of the core surface was changed by imbibition of low salinity water. However, different mechanisms contribute to the wettability alteration of porous rock surface which is explained in the following sections.
After the Amott test performed with distilled water (Test 1), the concentrations of Na+, K+, Ca2+ and Mg2+ in the imbibing water were 1.41, 0.15, 3.78 and 0.44 ppm (Fig. 9). This indicates that desorption of ions from the rock surface into the water occurred since no ions existed in the distilled water at the start of test. However, the ion concentration changes were very small, which is in good agreement with the small contact angle alteration in the aforementioned section.
Figure 10 shows the detailed ion concentration change percent in the imbibition fluids after Amott tests (Tests 2–5). In all tests, significant changes in ion concentrations were observed which clearly confirms the ion exchange during the spontaneous imbibition tests.
A considerable reduction in ions in the imbibing water can be explained by adsorption of ions on the core surface. This is in agreement with the contact angle measurements discussed in the previous section (see Fig. 7). Physical properties of the core surface were changed by precipitation of ions. As illustrated in Fig. 9, the concentrations of Na+ and Mg2+ were lower than those in the original low salinity water for all tests. For Test 2, the Ca2+ concentration was higher (about 55%) than that in the original imbibing water (i.e., 40-tdsw). This can be explained by dissolution of calcite in the low salinity water. In Test 2, the Ca2+ concentration in the imbibing water was the lowest compared with other low salinity water used, which may enhance the dissolution of calcite in water. Dissolution of calcite in the low salinity water may result in a change in pH of the imbibing water. Furthermore, lowering the dilution ratio (i.e., increasing water salinity) from optimum value has resulted in a higher adsorption of Ca2+ on the rock surface which was detected with a decrease in the Ca2+ concentration.
As mentioned above, Fig. 10 shows a significant increase in Ca2+ concentration in the imbibition solution for Test 2 which was conducted using 40-tdsw (brine with the lowest salinity) and this increase demonstrated that the mineral dissolution mechanism plays an important role in wettability alteration and also because of low salinity, ion adsorption is negligible. On the other hand, in Tests 4 and 5 with much higher salinity (10-tdsw and 5-tdsw), the initial Ca2+ concentration was very high and as a result, the ion adsorption mechanism, which is also believed to be contributing to wettability alteration, has caused a significant drop in Ca2+ concentration in the imbibing brine, but this high Ca2+ concentration has suppressed the chances of mineral dissolution. However, the highest recovery was achieved in Test 3 using 20-tdsw brine and a very small change in Ca2+ ion concentration was observed (Fig. 10). It could be concluded that at this level of salinity, both mechanisms, mineral dissolution and ion adsorption, are active and as a result, no significant change in Ca2+ concentration was observed since the ions produced by one mechanism are consumed by the other one. Hence, imbibition with 20-tdsw has led to the best recovery by keeping both contributing mechanisms active.
The pH values of the imbibition fluids were also measured before and after each test and are shown in Fig. 11. In all tests, the pH value of the imbibition fluid increased slightly after the test. Aksulu et al. (2012) stated that the pH was affected by the presence of calcite and anhydrite in the core plug structure (Aksulu et al. 2012). The highest pH difference was observed in Test 2. This is in agreement with previous analysis of ions of Test 2, the highest increase in Ca2+ concentration in the imbibing water. Clearly, the dissolution of calcite from the core surface into water affected the pH value as well as the cation concentration in the low salinity water. Oil droplets connected into the carbonate core surface were desorbed from the surface by increasing carbonate dissolution in the low salinity water. So, the pH enhancement led to an increase in oil recovery from the porous media.
It should be noted that with the pH increase, the zeta-potential of limestone is increased which means that the rock surface becomes more positive; hence, it is expected to recover more oil in higher pH differences (Mahani et al. 2015). However, in our tests, more oil was recovered in Test 3 (pH difference 0.8) compared with Test 2 with the highest pH difference value (pH difference 1.28). Despite the fact that the pH differences between two tests (Tests 2 and 3) are not very significant, it is concluded that there is no distinct relation between oil recovery and pH value which dictates the amount of positive charge on the rock surface.
Moreover, the pH change of low salinity water was not very significant compared with the considerable ion exchange during spontaneous imbibition tests. Therefore, ion exchange plays a more important role than pH in the low salinity spontaneous imbibition EOR for carbonate core plugs. Finally, the salting-in mechanism (Austad et al. 2007) is not considered in this study.
3.2 Temperature effect
Three Amott tests were conducted at elevated temperatures of 35, 55 and 75 °C to investigate the effect of temperature on oil recovery from carbonate core plugs by 20-tdsw (the optimum dilution ratio of sea water). There is no need to correct the oil production values due to the temperature rise since thermal expansion of oil is small.
It can be seen from Fig. 12 that there was a significant jump in oil recovery when the imbibition temperature increased from 55 to 75 °C. This highlights that the effect of temperature becomes more pronounced at higher temperatures. In addition, interaction between brine and the rock surface is enhanced by increasing the temperature. Moreover, the asphaltic and heavy molecules in crude oil which have been attached on the core surface can be more easily substituted by the brine at higher temperatures. Consequently, wettability alteration occurred faster and more efficiently at higher temperatures by removing connected oil from the core surface. In addition to the wettability alteration mechanism, viscosity reduction is considered as another mechanism due to an increase in temperature. However, wettability alteration is considered as the dominant mechanism compared with viscosity reduction since there is no significant reduction in viscosity from 35 to 55 °C and even less change from 55 to 75 °C (Table 3). Figure 8 shows that the ultimate oil recovery was 1.6% IOIP using distilled water (Test 1), but 13.9% IOIP using 20-tdsw (Test 3) at 75 °C. The oil recovery was very low in the Amott test with distilled water in which there was no impact of salinity and all oil recovery was due to temperature’s mechanism. Therefore, higher oil recovery in Test 3 was due to higher ion activity which consequently alters wettability condition more efficiently.
3.3 Permeability effect
An additional test was performed with a high-permeability core plug (Core 4, 182.25 mD) to investigate the effect of core permeability. As illustrated in Fig. 13, there was a significant difference in oil recovery between two tests conducted at 75 °C and 14.7 psi. Generally, a higher imbibition rate results from an increase in permeability. In a spontaneous imbibition process, oil production depends on suction of water and expulsion of the oil simultaneously into and out of the porous medium. Capillary forces act as the driving force to overcome resistance forces preventing oil extraction from the medium. For higher-permeability samples, water penetrates much more easily into pores in the media due to lower resistance forces, facilitating the production mechanisms to act inside the medium more efficiently. Therefore, oil production performance was much efficient in higher-permeability samples. This is in good agreement with findings of other researchers (Robbana et al. 2012; Shaker Shiran and Skauge 2012; Shehata and Nasr El-Din 2015).
Figure 13 also shows that the imbibition time required to obtain the ultimate oil recovery was shorter for the high-permeability core plug compared with the low-permeability ones. In this regard, spontaneous imbibition of low salinity water is more effective in high-permeability carbonate reservoirs in a short-term and long-term imbibition process.
The ion concentrations in the imbibing water were measured before and after each test and are listed in Table 6. In general, higher ion exchange occurred when the high-permeability core plug was immersed in the imbibing water compared with the low-permeability core plug except for Ca2+. So, calcite dissolution occurred more in the low-permeability core plug.
3.4 Connate water effect
Connate water plays an important role in oil reservoirs and has a significant effect on their behavior because it can form an intermediate layer between the rock surface and crude oil which is electrostatically attached to the surface; therefore, oil adsorption on the rock surface happens either directly (with no intermediate water layer or a negligibly thin one) or indirectly through a significantly thick layer of water (Brady and Thyne 2016). This layer of water can facilitate ion transfer between the imbibition fluid and the rock surface, resulting in a higher efficiency of low salinity effect. Figure 14 demonstrates these two types of adsorption.
In order to investigate the effect of connate water, an Amott test (Test 9) was conducted on a core which was primarily saturated with sea water (Persian Gulf water having 53,000 ppm salinity) and then flooded with oil until no water was produced, i.e., an irreducible water saturation was obtained. The experimental conditions were exactly the same as Test 3 (Table 5). Figure 15 shows a comparison between these two tests. The oil recovery increased from 14% to 28% IOIP when the connate water saturation increased from 0 to 25%. The existence of connate water would facilitate ion transport between the rock surface and brine and then alter the rock wettability effectively, making spontaneous imbibition of low salinity water more effective.
4 Conclusions
In this experimental study, low salinity spontaneous imbibition tests and contact angle measurements were conducted with diluted sea water on carbonate reservoir core samples to investigate the effects of salinity, temperature, permeability and connate water on oil recovery.
-
1.
20-fold dilution of sea water (20-tdsw) caused the highest oil recovery and the minimum contact angle. Brines with higher and lower salinities were less effective.
-
2.
Deionized water did not enhance oil recovery or reduce the contact angle.
-
3.
Surface ion exchange seemed to be the main mechanism of oil recovery enhancement. Mineral dissolution only acted as a secondary contributor.
-
4.
Higher temperature, higher permeability and the presence of connate water caused significant enhancements in the oil recovery.
References
Aksulu H, Håmsø D, Strand S, Puntervold T, Austad T. Evaluation of low-salinity enhanced oil recovery effects in sandstone: effects of the temperature and pH gradient. Energy Fuels. 2012;26(6):3497–503. https://doi.org/10.1021/ef300162n.
Al-Hadhrami HS, Blunt MJ. Thermally induced wettability alteration to improve oil recovery in fractured reservoirs. In: SPE/DOE improved oil recovery symposium, Tulsa. 2000. https://doi.org/10.2118/59289-MS.
Austad T, Strand S, Madland MV, Puntervold T, Korsnes RI. Seawater in chalk: an EOR and compaction fluid. In: International petroleum technology conference, Dubai. 2007. https://doi.org/10.2523/IPTC-11370-MS.
Austad T, RezaeiDoust A, Puntervold T. Chemical mechanism of low salinity water flooding in sandstone reservoirs. In: SPE improved oil recovery symposium, Tulsa, Oklahoma. 2010. https://doi.org/10.2118/129767-MS.
Brady PV, Thyne G. Functional wettability in carbonate reservoirs. Energy Fuels. 2016;30(11):9217–25. https://doi.org/10.1021/acs.energyfuels.6b01895.
Firoozabadi A. Recovery mechanisms in fractured reservoirs and field performance. J Can Pet Technol. 2000;39(11):13–7. https://doi.org/10.2118/00-11-DAS.
Green DW, Willhite GP. Enhanced oil recovery. Richardson: Henry L. Doherty Memorial Fund of AIME, Society of Petroleum Engineers. 1998.
Hatiboglu C, Babadagli T. Experimental analysis of primary and secondary oil recovery from matrix by counter-current diffusion and spontaneous imbibition. In: SPE annual technical conference and exhibition. 2004. https://doi.org/10.2118/90312-MS.
Hirasaki G, Zhang DL. Surface chemistry of oil recovery from fractured oil-wet carbonate formations. SPE J. 2004;9(02):151–62. https://doi.org/10.2118/88365-PA.
Kulathu S, Dandekar AY, Patil S, Khataniar S, editors. Low salinity cyclic water floods for enhanced oil recovery on Alaska North Slope. In: SPE Asia Pacific oil and gas conference and exhibition, Jakarta. 2013. https://doi.org/10.2118/165812-MS.
Lager A, Webb KJ, Black CJJ. Impact of brine chemistry on oil recovery. In: IOR 2007-14th European symposium on improved oil recovery. 2007.
Lager A, Webb KJ, Black CJJ, Singleton M, Sorbie KS. Low salinity oil recovery-an experimental investigation. Petrophysics. 2008a;49(01):28–35.
Lager A, Webb KJ, Collins IR, Richmond DM. LoSal™ enhanced oil recovery: evidence of enhanced oil recovery at the reservoir scale. In: SPE symposium on improved oil recovery, Tulsa. 2008b. https://doi.org/10.2118/113976-MS.
Lee SY, Webb KJ, Collins IR, Lager A, Clarke SM, O’Sullivan M. Low salinity oil recovery-increasing understanding of the underlying mechanisms. In: SPE improved oil recovery symposium, Tulsa. 2010. https://doi.org/10.2118/129722-MS.
Mahani H, Keya AL, Berg S, Bartels W-B, Nasralla R, Rossen WR. Insights into the mechanism of wettability alteration by low-salinity flooding (LSF) in carbonates. Energy Fuels. 2015;29(3):1352–67. https://doi.org/10.1021/ef5023847.
McGuire PL, Chatham JR, Paskvan FK, Sommer DM, Carini FH. Low salinity oil recovery: an exciting new EOR opportunity for Alaska’s North Slope. In: SPE western regional meeting Irvine, CA. 2005. https://doi.org/10.2118/93903-MS.
Morrow N, Buckley J. Improved oil recovery by low-salinity waterflooding. J Pet Technol. 2011;63(05):106–12. https://doi.org/10.2118/129421-JPT.
Nasralla RA, Alotaibi MB, Nasr-El-Din HA. Efficiency of oil recovery by low salinity water flooding in sandstone reservoirs. In: SPE Western North American region meeting, Anchorage. 2011. https://doi.org/10.2118/144602-MS.
Navratil KC. An experimental study of low salinity EOR effects on a core from the Yme field. Master thesis. University of Stavanger. 2012.
Patil SB, Dandekar AY, Patil S, Khataniar S. Low salinity brine injection for EOR on Alaska North Slope (ANS). In: International petroleum technology conference, Kuala Lumpur. 2008. https://doi.org/10.2523/IPTC-12004-MS.
Rezaeidoust A, Puntervold T, Strand S, Austad T. Smart water as wettability modifier in carbonate and sandstone: a discussion of similarities/differences in the chemical mechanisms. Energy Fuels. 2009;23(9):4479–85. https://doi.org/10.1021/ef900185q.
Rivet SM, Lake LW, Pope GA. A coreflood investigation of low-salinity enhanced oil recovery. In: SPE annual technical conference and exhibition, Florence. 2010. https://doi.org/10.2118/134297-MS.
Robbana E, Buikema TA, Mair C, Williams D, Mercer DJ, Webb KJ. Low salinity enhanced oil recovery—laboratory to day one field implementation—LoSal EOR into the clair ridge project. In: Abu Dhabi international petroleum conference and exhibition, Abu Dhabi. 2012. https://doi.org/10.2118/161750-MS.
Sari A, Xie Q, Chen Y, Saeedi A, Pooryousefy E. Drivers of low salinity effect in carbonate reservoirs. Energy Fuels. 2017;31(9):8951–8. https://doi.org/10.1021/acs.energyfuels.7b00966.
Schembre JM, Tang GQ, Kovscek AR. Wettability alteration and oil recovery by water imbibition at elevated temperatures. J Pet Sci Eng. 2006;52(1):131–48. https://doi.org/10.1016/j.petrol.2006.03.017.
Shaddel S, Tabatabae-Nejad SA. Alkali/surfactant improved low-salinity waterflooding. Transp Porous Media. 2015;106(3):621–42. https://doi.org/10.1007/s11242-014-0417-1.
Shaddel S, Hemmati M, Zamanian E, Moharrami NN. Core flood studies to evaluate efficiency of oil recovery by low salinity water flooding as a secondary recovery process. J Pet Sci Eng. 2014;4(1):47–56. https://doi.org/10.22078/jpst.2014.322.
Shaker Shiran B, Skauge A. Wettability and oil recovery by low salinity injection. In: SPE EOR conference at oil and gas West Asia, Muscat. 2012. https://doi.org/10.2118/155651-MS.
Shaker Shiran B, Skauge A. Enhanced oil recovery (EOR) by combined low salinity water/polymer flooding. Energy Fuels. 2013;27(3):1223–35. https://doi.org/10.1021/ef301538e.
Shariatpanahi SF, Hopkins P, Aksulu H, Strand S, Puntervold T, Austad T. Water based EOR by wettability alteration in dolomite. Energy Fuels. 2016;30(1):180–7. https://doi.org/10.1021/acs.energyfuels.5b02239.
Shehata AM, Nasr El-Din HA. Spontaneous imbibition study: effect of connate water composition on low-salinity waterflooding in sandstone reservoirs. In: SPE western regional meeting, Garden Grove. 2015. https://doi.org/10.2118/174063-MS.
Simjoo M, Pashaie H, Tabatabaienejad SA. Experimental study of spontaneous imbibition imposed by silica nanoparticle in carbonate cores. In: The 1st national conference on the development of oil and gas fields, Sharif University of Technology: CIVILICA. 2015.
Standnes DC, Austad T. Wettability alteration in chalk: 2. Mechanism for wettability alteration from oil-wet to water-wet using surfactants. J Pet Sci Eng. 2000;28(3):123–43. https://doi.org/10.1016/S0920-4105(00)00084-X.
Tang GQ, Morrow NR. Influence of brine composition and fines migration on crude oil/brine/rock interactions and oil recovery. J Pet Sci Eng. 1999;24(2):99–111. https://doi.org/10.1016/S0920-4105(99)00034-0.
Torrijos IDP, Puntervold T, Strand S, Austad T, Abdullah HI, Olsen K. Experimental study of the response time of the low-salinity enhanced oil recovery effect during secondary and tertiary low-salinity waterflooding. Energy Fuels. 2016;30(6):4733–9. https://doi.org/10.1021/acs.energyfuels.6b00641.
Wickramathilaka S, Howard JJ, Morrow NR, Buckley J. An experimental study of low salinity waterflooding and spontaneous imbibition. In: The 16th European symposium on improved oil recovery, Cambridge. 2011. https://doi.org/10.3997/2214-4609.201404795.
Zahid A, Shapiro AA, Skauge A. Experimental studies of low salinity water flooding carbonate: a new promising approach. In: SPE EOR conference at oil and gas West Asia, 16–18 April, Muscat. 2012. https://doi.org/10.2118/155625-MS.
Zhang P, Tweheyo MT, Austad T. Wettability alteration and improved oil recovery by spontaneous imbibition of seawater into chalk: impact of the potential determining ions Ca2+, Mg2+, and SO4 2−. Colloids Surf A Physicochem Eng Aspects. 2007;301(1):199–208. https://doi.org/10.1016/j.colsurfa.2006.12.058.
Acknowledgements
The authors acknowledge the National Iranian South Oil Company (NISOC) for generously funding the project as well as granting the permission to publish this paper. All the experimental works have been conducted in the Chemical Engineering Department at the Isfahan University of Technology, Isfahan, Iran.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Yan-Hua Sun
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Zaeri, M.R., Hashemi, R., Shahverdi, H. et al. Enhanced oil recovery from carbonate reservoirs by spontaneous imbibition of low salinity water. Pet. Sci. 15, 564–576 (2018). https://doi.org/10.1007/s12182-018-0234-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12182-018-0234-1