Abstract
Purpose of Review
The incidence of complications from prosthetic joint infection (PJI) is increasing, and treatment failure remains high. We review the current literature with a focus on Staphylococcus aureus pathogenesis and biofilm, as well as treatment challenges, and novel therapeutic strategies.
Recent Findings
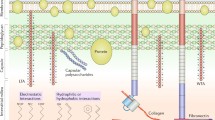
S. aureus biofilm creates a favorable environment that increases antibiotic resistance, impairs host immunity, and increases tolerance to nutritional deprivation. Secreted proteins from bacterial cells within the biofilm and the quorum-sensing agr system contribute to immune evasion. Additional immunoevasive properties of S. aureus include the formation of staphylococcal abscess communities (SACs) and canalicular invasion. Novel approaches to target biofilm and increase resistance to implant colonization include novel antibiotic therapy, immunotherapy, and local implant treatments.
Summary
Challenges remain given the diverse mechanisms developed by S. aureus to alter the host immune responses. Further understanding of these processes should provide novel therapeutic mechanisms to enhance eradication after PJI.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major Importance
Bryan AJ, Abdel MP, Sanders TL, Fitzgerald SF, Hanssen AD, Berry DJ. Irrigation and debridement with component retention for acute infection after hip arthroplasty: improved results with contemporary management. J Bone Joint Surg Am. 2017;99(23):2011–8. https://doi.org/10.2106/JBJS.16.01103.
Lora-Tamayo J, Murillo O, Iribarren JA, Soriano A, Sánchez-Somolinos M, Baraia-Etxaburu JM, et al. A large multicenter study of methicillin-susceptible and methicillin-resistant Staphylococcus aureus prosthetic joint infections managed with implant retention. Clin Infect Dis. 2013;56(2):182–94. https://doi.org/10.1093/cid/cis746.
Nodzo SR, Boyle KK, Spiro S, Nocon AA, Miller AO, Westrich GH. Success rates, characteristics, and costs of articulating antibiotic spacers for total knee periprosthetic joint infection. Knee. 2017;24(5):1175–81. https://doi.org/10.1016/j.knee.2017.05.016.
Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466(7):1710–5. https://doi.org/10.1007/s11999-008-0209-4.
Kaplan SL. Recent lessons for the management of bone and joint infections. J Inf Secur. 2014;68(Suppl 1):S51–6. https://doi.org/10.1016/j.jinf.2013.09.014.
Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2(2):95–108.
Flemming HC, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;8(9):623–33. https://doi.org/10.1038/nrmicro2415.
de Beer D, Stoodley P, Roe F, Lewandowski Z. Effects of biofilm structures on oxygen distribution and mass transport. Biotechnol Bioeng. 1994;43(11):1131–8.
•• Periasamy S, Joo HS, Duong AC, Bach TH, Tan VY, Chatterjee SS, et al. How Staphylococcus aureus biofilms develop their characteristic structure. Proc Natl Acad Sci U S A. 2012;109(4):1281–6. https://doi.org/10.1073/pnas.1115006109. Described the critical role of PSMs in biofilm structure and disassembly, which was driven by the agr quorum-sensing system.
Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284(5418):1318–22.
Mah TF, O'Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9(1):34–9.
Gristina AG, Hobgood CD, Webb LX, Myrvik QN. Adhesive colonization of biomaterials and antibiotic resistance. Biomaterials. 1987;8(6):423–6.
Jefferson KK, Goldmann DA, Pier GB. Use of confocal microscopy to analyze the rate of vancomycin penetration through Staphylococcus aureus biofilms. Antimicrob Agents Chemother. 2005;49(6):2467–73.
Zheng Z, Stewart PS. Penetration of rifampin through Staphylococcus epidermidis biofilms. Antimicrob Agents Chemother. 2002;46(3):900–3.
Anderl JN, Franklin MJ, Stewart PS. Role of antibiotic penetration limitation in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob Agents Chemother. 2000;44(7):1818–24.
Roberts ME, Stewart PS. Modelling protection from antimicrobial agents in biofilms through the formation of persister cells. Microbiology. 2005;151(Pt 1):75–80.
de la Fuente-Núñez C, Reffuveille F, Fernández L, Hancock RE. Bacterial biofilm development as a multicellular adaptation: antibiotic resistance and new therapeutic strategies. Curr Opin Microbiol. 2013;16(5):580–9. https://doi.org/10.1016/j.mib.2013.06.013.
Kahl B, Herrmann M, Everding AS, Koch HG, Becker K, Harms E, et al. Persistent infection with small colony variant strains of Staphylococcus aureus in patients with cystic fibrosis. J Infect Dis. 1998;177(4):1023–9.
Sendi P, Rohrbach M, Graber P, Frei R, Ochsner PE, Zimmerli W. Staphylococcus aureus small colony variants in prosthetic joint infection. Clin Infect Dis. 2006;43(8):961–7.
Garcia LG, Lemaire S, Kahl BC, Becker K, Proctor RA, Denis O, et al. Antibiotic activity against small-colony variants of Staphylococcus aureus: review of in vitro, animal and clinical data. J Antimicrob Chemother. 2013;68(7):1455–64. https://doi.org/10.1093/jac/dkt072.
Whiteley M, Bangera MG, Bumgarner RE, Parsek MR, Teitzel GM, Lory S, et al. Gene expression in Pseudomonas aeruginosa biofilms. Nature. 2001;413(6858):860–4.
Werner E, Roe F, Bugnicourt A, Franklin MJ, Heydorn A, Molin S, et al. Stratified growth in Pseudomonas aeruginosa biofilms. Appl Environ Microbiol. 2004;70(10):6188–96.
Deligianni E, Pattison S, Berrar D, Ternan NG, Haylock RW, Moore JE, et al. Pseudomonas aeruginosa cystic fibrosis isolates of similar RAPD genotype exhibit diversity in biofilm forming ability in vitro. BMC Microbiol. 2010;10:38. https://doi.org/10.1186/1471-2180-10-38.
Donlan RM, Piede JA, Heyes CD, Sanii L, Murga R, Edmonds P, et al. Model system for growing and quantifying Streptococcus pneumoniae biofilms in situ and in real time. Appl Environ Microbiol. 2004;70(8):4980–8.
Ceri H, Olson ME, Stremick C, Read RR, Morck D, Buret A. The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J Clin Microbiol. 1999;37(6):1771–6.
Yang L, Haagensen JA, Jelsbak L, Johansen HK, Sternberg C, Høiby N, et al. In situ growth rates and biofilm development of Pseudomonas aeruginosa populations in chronic lung infections. J Bacteriol. 2008;190(8):2767–76.
• Nishitani K, Sutipornpalangkul W, de Mesy Bentley KL, Varrone JJ, Bello-Irizarry SN, Ito H, et al. Quantifying the natural history of biofilm formation in vivo during the establishment of chronic implant-associated Staphylococcus aureus osteomyelitis in mice to identify critical pathogen and host factors. J Orthop Res. 2015;33(9):1311–9. https://doi.org/10.1002/jor.22907. The natural history of in vivo biofilm formation with multiple Staphylococcus aureus strains were examined in a clinically relevant murine model with an implant-associated infection.
Ulphani JS, Rupp ME. Model of Staphylococcus aureus central venous catheter-associated infection in rats. Lab Anim Sci. 1999;49(3):283–7.
Cirioni O, Giacometti A, Ghiselli R, Bergnach C, Orlando F, Mocchegiani F, et al. Pre-treatment of central venous catheters with the cathelicidin BMAP-28 enhances the efficacy of antistaphylococcal agents in the treatment of experimental catheter-related infection. Peptides. 2006;27(9):2104–10.
Geoghegan JA, Foster TJ. Cell wall-anchored surface proteins of Staphylococcus aureus: many proteins, multiple functions. Curr Top Microbiol Immunol. 2017;409:95–120. https://doi.org/10.1007/82_2015_5002.
Foster TJ. The remarkably multifunctional fibronectin binding proteins of Staphylococcus aureus. Eur J Clin Microbiol Infect Dis. 2016;35(12):1923–31.
Patti JM, Allen BL, McGavin MJ, Höök M. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu Rev Microbiol. 1994;48:585–617.
Archer NK, Mazaitis MJ, Costerton JW, Leid JG, Powers ME, Shirtliff ME. Staphylococcus aureus biofilms: properties, regulation, and roles in human disease. Virulence. 2011;2(5):445–59. https://doi.org/10.4161/viru.2.5.17724.
Ní Eidhin D, Perkins S, Francois P, Vaudaux P, Höök M, Foster TJ. Clumping factor B (ClfB), a new surface-located fibrinogen-binding adhesin of Staphylococcus aureus. Mol Microbiol. 1998;30(2):245–57.
Herman-Bausier P, Formosa-Dague C, Feuillie C, Valotteau C, Dufrêne YF. Forces guiding staphylococcal adhesion. J Struct Biol. 2017;197(1):65–9. https://doi.org/10.1016/j.jsb.2015.12.009.
O'Neill E, Pozzi C, Houston P, Humphreys H, Robinson DA, Loughman A, et al. A novel Staphylococcus aureus biofilm phenotype mediated by the fibronectin-binding proteins, FnBPA and FnBPB. J Bacteriol. 2008;190(11):3835–50. https://doi.org/10.1128/JB.00167-08.
Cardile AP, Sanchez CJ Jr, Samberg ME, Romano DR, Hardy SK, Wenke JC, et al. Human plasma enhances the expression of staphylococcal microbial surface components recognizing adhesive matrix molecules promoting biofilm formation and increases antimicrobial tolerance in vitro. BMC Res Notes. 2014;7:457. https://doi.org/10.1186/1756-0500-7-457.
Farnsworth CW, Schott EM, Jensen SE, Zukoski J, Benvie AM, Refaai MA, et al. Adaptive upregulation of clumping factor A (ClfA) by Staphylococcus aureus in the obese, type 2 diabetic host mediates increased virulence. Infect Immun. 2017;85(6):e01005–16. https://doi.org/10.1128/IAI.01005-16.
•• Wang Y, Cheng LI, Helfer DR, Ashbaugh AG, Miller RJ, Tzomides AJ, et al. Mouse model of hematogenous implant-related Staphylococcus aureus biofilm infection reveals therapeutic targets. Proc Natl Acad Sci U S A. 2017;114(26):E5094–102. https://doi.org/10.1073/pnas.1703427114. The authors developed a murine hematogenous implant-related infection model and demonstrated that a combination of neutralizing human monoclonal antibodies against α-toxin and ClfA inhibited in vitro biofilm formation, propensity for infection in vivo, and reduced bacterial burden in vivo.
Hammer ND, Skaar EP. Molecular mechanisms of Staphylococcus aureus iron acquisition. Annu Rev Microbiol. 2011;65:129–47. https://doi.org/10.1146/annurev-micro-090110-102851.
Clarke SR, Mohamed R, Bian L, Routh AF, Kokai-Kun JF, Mond JJ, et al. The Staphylococcus aureus surface protein IsdA mediates resistance to innate defenses of human skin. Cell Host Microbe. 2007;1(3):199–212.
Missineo A, Di Poto A, Geoghegan JA, Rindi S, Heilbronner S, Gianotti V, et al. IsdC from Staphylococcus lugdunensis induces biofilm formation under low-iron growth conditions. Infect Immun. 2014;82(6):2448–59. https://doi.org/10.1128/IAI.01542-14.
Zapotoczna M, Jevnikar Z, Miajlovic H, Kos J, Foster TJ. Iron-regulated surface determinant B (IsdB) promotes Staphylococcus aureus adherence to and internalization by non-phagocytic human cells. Cell Microbiol. 2013;15(6):1026–41. https://doi.org/10.1111/cmi.12097.
Shopsin B, Gomez M, Montgomery SO, Smith DH, Waddington M, Dodge DE, et al. Evaluation of protein A gene polymorphic region DNA sequencing for typing of Staphylococcus aureus strains. J Clin Microbiol. 1999;37(11):3556–63.
Muthukrishnan G, Quinn GA, Lamers RP, Diaz C, Cole AL, Chen S, et al. Exoproteome of Staphylococcus aureus reveals putative determinants of nasal carriage. J Proteome Res. 2011;10(4):2064–78. https://doi.org/10.1021/pr200029r.
Cole AL, Muthukrishnan G, Chong C, Beavis A, Eade CR, Wood MP, et al. Host innate inflammatory factors and staphylococcal protein A influence the duration of human Staphylococcus aureus nasal carriage. Mucosal Immunol. 2016;9(6):1537–48. https://doi.org/10.1038/mi.2016.2.
Gómez MI, Lee A, Reddy B, Muir A, Soong G, Pitt A, et al. Staphylococcus aureus protein A induces airway epithelial inflammatory responses by activating TNFR1. Nat Med. 2004;10(8):842–8.
Silverman GJ, Cary S, Graille M, Curtiss VE, Wagenknecht R, Luo L, et al. A B-cell superantigen that targets B-1 lymphocytes. Curr Top Microbiol Immunol. 2000;252:251–63.
Graille M, Stura EA, Corper AL, Sutton BJ, Taussig MJ, Charbonnier JB, et al. Crystal structure of a Staphylococcus aureus protein A domain complexed with the Fab fragment of a human IgM antibody: structural basis for recognition of B-cell receptors and superantigen activity. Proc Natl Acad Sci U S A. 2000;97(10):5399–404.
Ricklin D, Tzekou A, Garcia BL, Hammel M, McWhorter WJ, Sfyroera G, et al. A molecular insight into complement evasion by the staphylococcal complement inhibitor protein family. J Immunol. 2009;183(4):2565–74. https://doi.org/10.4049/jimmunol.0901443.
Merino N, Toledo-Arana A, Vergara-Irigaray M, Valle J, Solano C, Calvo E, et al. Protein A-mediated multicellular behavior in Staphylococcus aureus. J Bacteriol. 2009;191(3):832–43. https://doi.org/10.1128/JB.01222-08.
Mendoza Bertelli A, Delpino MV, Lattar S, Giai C, Llana MN, Sanjuan N, et al. Staphylococcus aureus protein A enhances osteoclastogenesis via TNFR1 and EGFR signaling. Biochim Biophys Acta. 2016;1862(10):1975–83. https://doi.org/10.1016/j.bbadis.2016.07.016.
Claro T, Widaa A, O'Seaghdha M, Miajlovic H, Foster TJ, O'Brien FJ, et al. Staphylococcus aureus protein A binds to osteoblasts and triggers signals that weaken bone in osteomyelitis. PLoS One. 2011;6(4):e18748. https://doi.org/10.1371/journal.pone.0018748.
Widaa A, Claro T, Foster TJ, O'Brien FJ, Kerrigan SW. Staphylococcus aureus protein A plays a critical role in mediating bone destruction and bone loss in osteomyelitis. PLoS One. 2012;7(7):e40586. https://doi.org/10.1371/journal.pone.0040586.
Claro T, Widaa A, McDonnell C, Foster TJ, O'Brien FJ, Kerrigan SW. Staphylococcus aureus protein A binding to osteoblast tumour necrosis factor receptor 1 results in activation of nuclear factor kappa B and release of interleukin-6 in bone infection. Microbiology. 2013;159(Pt 1):147–54. https://doi.org/10.1099/mic.0.063016-0.
Cassat JE, Hammer ND, Campbell JP, Benson MA, Perrien DS, Mrak LN, et al. A secreted bacterial protease tailors the Staphylococcus aureus virulence repertoire to modulate bone remodeling during osteomyelitis. Cell Host Microbe. 2013;13(6):759–72. https://doi.org/10.1016/j.chom.2013.05.003.
Brady RA, Leid JG, Camper AK, Costerton JW, Shirtliff ME. Identification of Staphylococcus aureus proteins recognized by the antibody-mediated immune response to a biofilm infection. Infect Immun. 2006;74(6):3415–26.
Flammier S, Rasigade JP, Badiou C, Henry T, Vandenesch F, Laurent F, et al. Human monocyte-derived osteoclasts are targeted by staphylococcal pore-forming toxins and superantigens. PLoS One. 2016;11(3):e0150693. https://doi.org/10.1371/journal.pone.0150693.
Biswas R, Voggu L, Simon UK, Hentschel P, Thumm G, Götz F. Activity of the major staphylococcal autolysin Atl. FEMS Microbiol Lett. 2006;259(2):260–8.
Weidenmaier C, Peschel A. Teichoic acids and related cell-wall glycopolymers in Gram-positive physiology and host interactions. Nat Rev Microbiol. 2008;6(4):276–87. https://doi.org/10.1038/nrmicro1861.
Chavakis T, Wiechmann K, Preissner KT, Herrmann M. Staphylococcus aureus interactions with the endothelium: the role of bacterial “secretable expanded repertoire adhesive molecules” (SERAM) in disturbing host defense systems. Thromb Haemost. 2005;94(2):278–85.
Rigby KM, DeLeo FR. Neutrophils in innate host defense against Staphylococcus aureus infections. Semin Immunopathol. 2012;34(2):237–59. https://doi.org/10.1007/s00281-011-0295-3.
•• Wang R, Braughton KR, Kretschmer D, Bach TH, Queck SY, Li M, et al. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med. 2007;13(12):1510–4. Identified PSMs as a major determinant of S. aureus virulence including leukocyte lysis. Expression of PSMs was found to be tightly regulated through the agr quorum-sensing system.
Scherr TD, Roux CM, Hanke ML, Angle A, Dunman PM, Kielian T. Global transcriptome analysis of Staphylococcus aureus biofilms in response to innate immune cells. Infect Immun. 2013;81(12):4363–76. https://doi.org/10.1128/IAI.00819-13.
Clauditz A, Resch A, Wieland KP, Peschel A, Götz F. Staphyloxanthin plays a role in the fitness of Staphylococcus aureus and its ability to cope with oxidative stress. Infect Immun. 2006;74(8):4950–3.
• Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–5. Described the characteristics and release of neutrophil extracellular traps (NETs), which are granule proteins and chromatin that are released extracellularly to degrade virulence factors and immobilize bacteria for phagocytic clearance.
Lu T, Kobayashi SD, Quinn MT, Deleo FR. A NET Outcome. Front Immunol. 2012;3:365. https://doi.org/10.3389/fimmu.2012.00365.
•• Thammavongsa V, Missiakas DM, Schneewind O. Staphylococcus aureus degrades neutrophil extracellular traps to promote immune cell death. Science. 2013;342(6160):863–6. https://doi.org/10.1126/science.1242255. Described a novel mechanism of immune evasion by which S. aureus degrades neutrophil NETs to deoxyadenosine, which is toxic to host immune cells.
• Cheng AG, DeDent AC, Schneewind O, Missiakas D. A play in four acts: Staphylococcus aureus abscess formation. Trends Microbiol. 2011;19(5):225–32. https://doi.org/10.1016/j.tim.2011.01.007. A review describing the establishment of S. aureus infection and staphylococcal abscess community (SAC) formation and its effect on host immunity.
Gries CM, Kielian T. Staphylococcal biofilms and immune polarization during prosthetic joint infection. J Am Acad Orthop Surg. 2017;25(Suppl 1):S20–4. https://doi.org/10.5435/JAAOS-D-16-00636.
• Thurlow LR, Hanke ML, Fritz T, Angle A, Aldrich A, Williams SH, et al. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J Immunol. 2011;186(11):6585–96. https://doi.org/10.4049/jimmunol.1002794. Using a mouse model of catheter-associated infection, the authors showed that S. aureus biofilm was capable of attenuating inflammatory cytokine production and skewing the immune response towards a pro-fibrotic M2 macrophage phenotype.
Scherr TD, Heim CE, Morrison JM, Kielian T. Hiding in plain sight: interplay between staphylococcal biofilms and host immunity. Front Immunol. 2014;5:37. https://doi.org/10.3389/fimmu.2014.00037.
Scherr TD, Hanke ML, Huang O, James DB, Horswill AR, Bayles KW, et al. Staphylococcus aureus biofilms induce macrophage dysfunction through leukocidin AB and alpha-toxin. MBio. 2015;6(4):e01021–15. https://doi.org/10.1128/mBio.01021-15.
• Heim CE, Vidlak D, Scherr TD, Kozel JA, Holzapfel M, Muirhead DE, et al. Myeloid-derived suppressor cells contribute to Staphylococcus aureus orthopedic biofilm infection. J Immunol. 2014;192(8):3778–92. https://doi.org/10.4049/jimmunol.1303408. In a murine model of prosthetic joint infection (PJI), the authors demonstrated that MDSCs actively suppressed T cell recruitment and proinflammatory cytokine production at the site of infection, thereby facilitating S. aureus biofilm persistence and proliferation.
Heim CE, Vidlak D, Kielian T. Interleukin-10 production by myeloid-derived suppressor cells contributes to bacterial persistence during Staphylococcus aureus orthopedic biofilm infection. J Leukoc Biol. 2015;98(6):1003–13. https://doi.org/10.1189/jlb.4VMA0315-125RR.
Heim CE, Vidlak D, Scherr TD, Hartman CW, Garvin KL, Kielian T. IL-12 promotes myeloid-derived suppressor cell recruitment and bacterial persistence during Staphylococcus aureus orthopedic implant infection. J Immunol. 2015;194(8):3861–72. https://doi.org/10.4049/jimmunol.1402689.
Peng KT, Hsieh CC, Huang TY, Chen PC, Shih HN, Lee MS, et al. Staphylococcus aureus biofilm elicits the expansion, activation and polarization of myeloid-derived suppressor cells in vivo and in vitro. PLoS One. 2017;12(8):e0183271. https://doi.org/10.1371/journal.pone.0183271.
Bröker BM, Mrochen D, Péton V. The T cell response to Staphylococcus aureus. Pathogens. 2016;5(1):E31. https://doi.org/10.3390/pathogens5010031.
Peck A, Mellins ED. Precarious balance: Th17 cells in host defense. Infect Immun. 2010;78(1):32–8. https://doi.org/10.1128/IAI.00929-09.
Jensen LK, Jensen HE, Koch J, Bjarnsholt T, Eickhardt S, Shirtliff M. Specific antibodies to Staphylococcus aureus biofilm are present in serum from pigs with osteomyelitis. In Vivo. 2015;29(5):555–60.
Prabhakara R, Harro JM, Leid JG, Keegan AD, Prior ML, Shirtliff ME. Suppression of the inflammatory immune response prevents the development of chronic biofilm infection due to methicillin-resistant Staphylococcus aureus. Infect Immun. 2011;79(12):5010–8. https://doi.org/10.1128/IAI.05571-11.
Holtfreter S, Nguyen TT, Wertheim H, Steil L, Kusch H, Truong QP, et al. Human immune proteome in experimental colonization with Staphylococcus aureus. Clin Vaccine Immunol. 2009;16(11):1607–14. https://doi.org/10.1128/CVI.00263-09.
Holtfreter S, Kolata J, Bröker BM. Towards the immune proteome of Staphylococcus aureus—the anti-S. aureus antibody response. Int J Med Microbiol. 2010;300(2–3):176–92. https://doi.org/10.1016/j.ijmm.2009.10.002.
Verkaik NJ, Boelens HA, de Vogel CP, Tavakol M, Bode LG, Verbrugh HA, et al. Heterogeneity of the humoral immune response following Staphylococcus aureus bacteremia. Eur J Clin Microbiol Infect Dis. 2010;29(5):509–18. https://doi.org/10.1007/s10096-010-0888-0.
Verkaik NJ, Lebon A, de Vogel CP, Hooijkaas H, Verbrugh HA, Jaddoe VW, et al. Induction of antibodies by Staphylococcus aureus nasal colonization in young children. Clin Microbiol Infect. 2010;16(8):1312–7. https://doi.org/10.1111/j.1469-0691.2009.03073.x.
• Nishitani K, Beck CA, Rosenberg AF, Kates SL, Schwarz EM, Daiss JL. A diagnostic serum antibody test for patients with Staphylococcus aureus osteomyelitis. Clin Orthop Relat Res. 2015;473(9):2735–49. https://doi.org/10.1007/s11999-015-4354-2. Described the serum host antibody response in patients with deep muscolskeletal infection with S. aureus versus healthy controls. No single titer yielded diagnostic significance; however, using a combination of host antibodies may provide diagnostic utility for ongoing infection.
Novick RP, Geisinger E. Quorum sensing in staphylococci. Annu Rev Genet. 2008;42:541–64. https://doi.org/10.1146/annurev.genet.42.110807.091640.
Boles BR, Horswill AR. Staphylococcal biofilm disassembly. Trends Microbiol. 2011;19(9):449–55. https://doi.org/10.1016/j.tim.2011.06.004.
Mann EE, Rice KC, Boles BR, Endres JL, Ranjit D, Chandramohan L, et al. Modulation of eDNA release and degradation affects Staphylococcus aureus biofilm maturation. PLoS One. 2009;4(6):e5822. https://doi.org/10.1371/journal.pone.0005822.
Vuong C, Saenz HL, Götz F, Otto M. Impact of the agr quorum-sensing system on adherence to polystyrene in Staphylococcus aureus. J Infect Dis. 2000;182(6):1688–93.
Vuong C, Kocianova S, Yao Y, Carmody AB, Otto M. Increased colonization of indwelling medical devices by quorum-sensing mutants of Staphylococcus epidermidis in vivo. J Infect Dis. 2004;190(8):1498–505.
Balasubramanian D, Ohneck EA, Chapman J, Weiss A, Kim MK, Reyes-Robles T, et al. Staphylococcus aureus coordinates leukocidin expression and pathogenesis by sensing metabolic fluxes via RpiRc. MBio. 2016;7(3):e00818–6. https://doi.org/10.1128/mBio.00818-16.
Paharik AE, Horswill AR. The staphylococcal biofilm: adhesins, regulation and host response. Microbiol Spectr. 2016;4(2) https://doi.org/10.1128/microbiolspec.VMBF-0022-2015.
Azzam K, McHale K, Austin M, Purtill JJ, Parvizi J. Outcome of a second two-stage reimplantation for periprosthetic knee infection. Clin Orthop Relat Res. 2009;467(7):1706–14. https://doi.org/10.1007/s11999-009-0739-4.
Aynardi M, Pulido L, Parvizi J, Sharkey PF, Rothman RH. Early mortality after modern total hip arthroplasty. Clin Orthop Relat Res. 2009;467(1):213–8. https://doi.org/10.1007/s11999-008-0528-5.
Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350(14):1422–9.
•• de Mesy Bentley KL, Trombetta R, Nishitani K, Bello-Irizarry SN, Ninomiya M, Zhang L, et al. Evidence of Staphylococcus aureus deformation, proliferation, and migration in canaliculi of live cortical bone in murine models of osteomyelitis. J Bone Miner Res. 2017;32(5):985–90. https://doi.org/10.1002/jbmr.3055. Used a murine implant-associated infection model to show that S. aureus was able to deform, enter the canalicular system via asymmetric binary fission, and migrate toward osteocyte lacunae via proliferation at the leading edge, providing a novel reservoir to protect S. aureus from host immunity.
de Mesy Bentley KL, MacDonald A, Schwarz EM, Oh I. Chronic osteomyelitis with Staphylococcus aureus deformation in submicron canaliculi of osteocytes: a case report. JBJS Case Connect. 2018;8:e8. https://doi.org/10.2106/JBJS.CC.17.00154.
Nishitani K, Bello-Irizarry SN, de Mesy Bentley K, Daiss JL, Schwarz EM. The role of the immune system and bone cells in acute and chronic osteomyelitis. Osteoimmunology. 2016; 2nd ed (Chapter 16):283–95.
Pollitt EJ, Crusz SA, Diggle SP. Staphylococcus aureus forms spreading dendrites that have characteristics of active motility. Sci Rep. 2015;5:17698. https://doi.org/10.1038/srep17698.
Tuchscherr L, Heitmann V, Hussain M, Viemann D, Roth J, von Eiff C, et al. Staphylococcus aureus small-colony variants are adapted phenotypes for intracellular persistence. J Infect Dis. 2010;202(7):1031–40. https://doi.org/10.1086/656047.
Tsompanidou E, Denham EL, van Dijl JM. Phenol-soluble modulins, hellhounds from the staphylococcal virulence-factor pandemonium. Trends Microbiol. 2013;21(7):313–5. https://doi.org/10.1016/j.tim.2013.04.007.
Kaito C, Sekimizu K. Colony spreading in Staphylococcus aureus. J Bacteriol. 2007;189(6):2553–7.
Cheng AG, Kim HK, Burts ML, Krausz T, Schneewind O, Missiakas DM. Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues. FASEB J. 2009;23(10):3393–404. https://doi.org/10.1096/fj.09-135467.
Jørgensen NP, Skovdal SM, Meyer RL, Dagnæs-Hansen F, Fuursted K, Petersen E. Rifampicin-containing combinations are superior to combinations of vancomycin, linezolid and daptomycin against Staphylococcus aureus biofilm infection in vivo and in vitro. Pathog Dis. 2016;74(4):ftw019. https://doi.org/10.1093/femspd/ftw019.
Niska JA, Shahbazian JH, Ramos RI, Francis KP, Bernthal NM, Miller LS. Vancomycin-rifampin combination therapy has enhanced efficacy against an experimental Staphylococcus aureus prosthetic joint infection. Antimicrob Agents Chemother. 2013;57(10):5080–6. https://doi.org/10.1128/AAC.00702-13.
Holmberg A, Thórhallsdóttir VG, Robertsson O, W-Dahl A, Stefánsdóttir A. 75% success rate after open debridement, exchange of tibial insert, and antibiotics in knee prosthetic joint infections. Acta Orthop. 2015;86(4):457–62. https://doi.org/10.3109/17453674.2015.1026756.
Puhto AP, Puhto T, Niinimäki T, Ohtonen P, Leppilahti J, Syrjälä H. Predictors of treatment outcome in prosthetic joint infections treated with prosthesis retention. Int Orthop. 2015;39(9):1785–91. https://doi.org/10.1007/s00264-015-2819-2.
Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-body infection (FBI) study group. JAMA. 1998;279(19):1537–41.
Jørgensen NP, Zobek N, Dreier C, Haaber J, Ingmer H, Larsen OH, et al. Streptokinase treatment reverses biofilm-associated antibiotic resistance in Staphylococcus aureus. Microorganisms. 2016;4(3):E36. https://doi.org/10.3390/microorganisms4030036.
Molina-Manso D, del Prado G, Ortiz-Pérez A, Manrubia-Cobo M, Gómez-Barrena E, Cordero-Ampuero J, et al. In vitro susceptibility to antibiotics of staphylococci in biofilms isolated from orthopaedic infections. Int J Antimicrob Agents. 2013;41(6):521–3. https://doi.org/10.1016/j.ijantimicag.2013.02.018.
Urish KL, DeMuth PW, Kwan BW, Craft DW, Ma D, Haider H, et al. Antibiotic-tolerant Staphylococcus aureus biofilm persists on arthroplasty materials. Clin Orthop Relat Res. 2016;474(7):1649–56. https://doi.org/10.1007/s11999-016-4720-8.
Bhattacharya M, Wozniak DJ, Stoodley P, Hall-Stoodley L. Prevention and treatment of Staphylococcus aureus biofilms. Expert Rev Anti-Infect Ther. 2015;13(12):1499–516. https://doi.org/10.1586/14787210.2015.1100533.
• Fowler VG, Allen KB, Moreira ED, Moustafa M, Isgro F, Boucher HW, et al. Effect of an investigational vaccine for preventing Staphylococcus aureus infections after cardiothoracic surgery: a randomized trial. JAMA. 2013;309(13):1368–78. https://doi.org/10.1001/jama.2013.3010. Phase 2b/3 trial of vaccine from Merck (V710), targeting IsdB in patients undergoing cardiothoracic surgery. V710 failed to reduce infection rates or mortality. Moreover, patients who did get infected were more likely to die in the vaccine group, suggesting that this vaccine may actually impair host immunity against sepsis.
McNeely TB, Shah NA, Fridman A, Joshi A, Hartzel JS, Keshari RS, et al. Mortality among recipients of the Merck V710 Staphylococcus aureus vaccine after postoperative S. aureus infections: an analysis of possible contributing host factors. Hum Vaccin Immunother. 2014;10(12):3513–6. https://doi.org/10.4161/hv.34407.
Shinefield H, Black S, Fattom A, Horwith G, Rasgon S, Ordonez J, et al. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N Engl J Med. 2002;346(7):491–6.
Estellés A, Woischnig AK, Liu K, Stephenson R, Lomongsod E, Nguyen D, et al. A high-affinity native human antibody disrupts biofilm from Staphylococcus aureus Bacteria and potentiates antibiotic efficacy in a mouse implant infection model. Antimicrob Agents Chemother. 2016;60(4):2292–301. https://doi.org/10.1128/AAC.02588-15.
Varrone JJ, de Mesy Bentley KL, Bello-Irizarry SN, Nishitani K, Mack S, Hunter JG, et al. Passive immunization with anti-glucosaminidase monoclonal antibodies protects mice from implant-associated osteomyelitis by mediating opsonophagocytosis of Staphylococcus aureus megaclusters. J Orthop Res. 2014;32(10):1389–96. https://doi.org/10.1002/jor.22672.
• Yokogawa N, Ishikawa M, Nishitani K, Beck CA, Tsuchiya H, Mesfin A, et al. Immunotherapy synergizes with debridement and antibiotic therapy in a murine 1-stage exchange model of MRSA implant-associated osteomyelitis. J Orthop Res. 2018; https://doi.org/10.1002/jor.23801. Using a murine implant-associated infection model, the authors found that immunotherapy with anti-Gmd monoclonal antibodies inhibited SACs while vancomycin reduced CFUs on the implant. Combination therapy provided the best results in soft tissue and implant infection control.
•• Lehar SM, Pillow T, Xu M, Staben L, Kajihara KK, Vandlen R, et al. Novel antibody-antibiotic conjugate eliminates intracellular S. aureus. Nature. 2015;527(7578):323–8. https://doi.org/10.1038/nature16057. The authors created an antibody-antibiotic conjugate (AAC) that consisted of a monoclonal antibody that recognizes the alpha-O-linked N-acetylglucosamine sugars on wall teichoic acids (WTAs) bound to rifamycin class derivative antibiotic to target intracellular S. aureus . This AAC demonstrated improved results versus systemic vancomycin alone in a murine MRSA hematogenous infection model.
Hogan S, Zapotoczna M, Stevens NT, Humphreys H, O’Gara JP, O'Neill E. Potential use of targeted enzymatic agents in the treatment of Staphylococcus aureus biofilm-related infections. J Hosp Infect. 2017;96(2):177–82. https://doi.org/10.1016/j.jhin.2017.02.008.
Hogan S, O’Gara JP, O’Neill E. Novel treatment of Staphylococcus aureus device-related infections using fibrinolytic agents. Antimicrob Agents Chemother. 2018;62(2):e02008–17. https://doi.org/10.1128/AAC.02008-17.
Lauderdale KJ, Malone CL, Boles BR, Morcuende J, Horswill AR. Biofilm dispersal of community-associated methicillin-resistant Staphylococcus aureus on orthopedic implant material. J Orthop Res. 2010;28(1):55–61. https://doi.org/10.1002/jor.20943.
Hardes J, von Eiff C, Streitbuerger A, Balke M, Budny T, Henrichs MP, et al. Reduction of periprosthetic infection with silver-coated megaprostheses in patients with bone sarcoma. J Surg Oncol. 2010;101(5):389–95. https://doi.org/10.1002/jso.21498.
• Wafa H, Grimer RJ, Reddy K, Jeys L, Abudu A, Carter SR, et al. Retrospective evaluation of the incidence of early periprosthetic infection with silver-treated endoprostheses in high-risk patients: case-control study. Bone Joint J. 2015;97-B(2):252–7. https://doi.org/10.1302/0301-620X.97B2.34554. A case-control clinical study using silver-coated megaprosthesis (Alguna). The silver-coated prosthesis had reduced rates of postoperative infection (11.8 versus 22.4%) and had improved success after debridement and implant retention relative to titanium implants.
Alt V. Antimicrobial coated implants in trauma and orthopaedics—a clinical review and risk-benefit analysis. Injury. 2017;48(3):599–607. https://doi.org/10.1016/j.injury.2016.12.011.
Kollef MH, Afessa B, Anzueto A, Veremakis C, Kerr KM, Margolis BD, et al. Silver-coated endotracheal tubes and incidence of ventilator-associated pneumonia: the NASCENT randomized trial. JAMA. 2008;300(7):805–13. https://doi.org/10.1001/jama.300.7.805.
Schierholz JM, Lucas LJ, Rump A, Pulverer G. Efficacy of silver-coated medical devices. J Hosp Infect. 1998;40(4):257–62.
van Hengel IAJ, Riool M, Fratila-Apachitei LE, Witte-Bouma J, Farrell E, Zadpoor AA, et al. Selective laser melting porous metallic implants with immobilized silver nanoparticles kill and prevent biofilm formation by methicillin-resistant Staphylococcus aureus. Biomaterials. 2017;140:1–15. https://doi.org/10.1016/j.biomaterials.2017.02.030.
Kucharíková S, Gerits E, De Brucker K, Braem A, Ceh K, Majdič G, et al. Covalent immobilization of antimicrobial agents on titanium prevents Staphylococcus aureus and Candida albicans colonization and biofilm formation. J Antimicrob Chemother. 2016;71(4):936–45. https://doi.org/10.1093/jac/dkv437.
Diefenbeck M, Schrader C, Gras F, Mückley T, Schmidt J, Zankovych S, et al. Gentamicin coating of plasma chemical oxidized titanium alloy prevents implant-related osteomyelitis in rats. Biomaterials. 2016;101:156–64. https://doi.org/10.1016/j.biomaterials.2016.05.039.
Drago L, Boot W, Dimas K, Malizos K, Hänsch GM, Stuyck J, et al. Does implant coating with antibacterial-loaded hydrogel reduce bacterial colonization and biofilm formation in vitro? Clin Orthop Relat Res. 2014;472(11):3311–23. https://doi.org/10.1007/s11999-014-3558-1.
Giavaresi G, Meani E, Sartori M, Ferrari A, Bellini D, Sacchetta AC, et al. Efficacy of antibacterial-loaded coating in an in vivo model of acutely highly contaminated implant. Int Orthop. 2014;38(7):1505–12. https://doi.org/10.1007/s00264-013-2237-2.
Jennings JA, Carpenter DP, Troxel KS, Beenken KE, Smeltzer MS, Courtney HS, et al. Novel antibiotic-loaded point-of-care implant coating inhibits biofilm. Clin Orthop Relat Res. 2015;473(7):2270–82. https://doi.org/10.1007/s11999-014-4130-8.
Ehrensberger MT, Tobias ME, Nodzo SR, Hansen LA, Luke-Marshall NR, Cole RF, et al. Cathodic voltage-controlled electrical stimulation of titanium implants as treatment for methicillin-resistant Staphylococcus aureus periprosthetic infections. Biomaterials. 2015;41:97–105. https://doi.org/10.1016/j.biomaterials.2014.11.013.
Leary JT, Werger MM, Broach WH, Shaw LN, Santoni BG, Bernasek TL, et al. Complete eradication of biofilm from orthopedic materials. J Arthroplast. 2017;32(8):2513–8. https://doi.org/10.1016/j.arth.2017.03.050.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Edward M Schwarz reports grants from NIH and AOTrauma during the conduct of the study and personal fees and other from Telephus, LLC, outside the submitted work. In addition, Dr. Schwarz has a patent on passive immunization and diagnostics for S. aureus licensed to Telephus, LLC.
The other authors declare that they have no conflicts of interest.
EMS has patents related to this work. EMS has received financial compensation and stock from Telephus Medical LLC.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Grant Sponsors
National Institutes of Health (EMS), Grant Numbers P30 AR069655 and P50 AR07200 (EMS), and AOTrauma Clinical Priority Program (EMS).
Additional information
This article is part of the Topical Collection on Prosthetic Joint Infection
Rights and permissions
About this article
Cite this article
Ricciardi, B.F., Muthukrishnan, G., Masters, E. et al. Staphylococcus aureus Evasion of Host Immunity in the Setting of Prosthetic Joint Infection: Biofilm and Beyond. Curr Rev Musculoskelet Med 11, 389–400 (2018). https://doi.org/10.1007/s12178-018-9501-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12178-018-9501-4