Abstract
Knowledge about biochemical, structural and physiological aspects, and properties regarding the skeletal muscle has been widely obtained in the last decades. Muscle disorders, mainly represented in neuromuscular clinical practice by acquired and hereditary myopathies, are well-recognized and frequently diagnosed in practice. Most clinical complaints and biochemical characterizations of each myopathy depends on the appropriate knowledge and interpretation of pathological findings and their comparison with normal muscle findings. Great improvement has been obtained in the last decades mainly involving the mechanisms of normal muscle architecture and physiological function in the healthy individuals. Genetic mechanisms have also been widely studied. We provide an extensive literature review involving current knowledge regarding muscle cell structure and function and embryological and regenerative processes linked to muscle lesion. An updated comprehensive description involving the main nuclear genomic regulatory mechanisms of muscle regeneration and embryogenesis is provided in this review.
Similar content being viewed by others
References
McNally EM, Pytel P. Muscle diseases: the muscular dystrophies. Annu Rev Pathol. 2007;2:87–109.
Reed UC. Congenital muscular dystrophy. Part II: a review of pathogenesis and therapeutic perspectives. Arq Neuropsiquiatr. 2009;67(2A):343–62.
Gundersen K. Excitation-transcription coupling in skeletal muscle: the molecular pathways of exercise. Biol Rev Camb Philos Soc. 2011;86(3):564–600.
Kaplan JC, Hamroun D. The 2015 version of the gene table of monogenic neuromuscular disorders (nuclear genome). Neuromuscul Disord. 2014;24(12):1123–53.
Cotta A, Carvalho E, Cunha-Júnior AL, Paim JF, Navarro MM, Valicek J, et al. Common recessive limb girdle muscular dystrophies differential diagnosis: why and how? Arq Neuropsiquiatr. 2014;72(9):721–34.
Merrison AFA, Hanna MG. The bare essentials: muscle disease. Pract Neurol. 2009;9(1):54–65.
Tobon A. Metabolic myopathies. Continuum (Minneap Minn). 2013;19(6):1571–97.
Iannaccone ST, Castro D. Congenital muscular dystrophies and congenital myopathies. Continuum (Minneap Minn). 2013;19(6):1509–34.
Wicklund MP. The muscular dystrophies. Continuum (Minneap Minn). 2013;19(6):1535–70.
Taivassalo T, Reddy H, Matthews PM. Muscle responses to exercise in health and disease. Neurol Clin. 2000;18(1):15–34.
Favier FB, Benoit H, Freyssenet D. Cellular and molecular events controlling skeletal muscle mass in response to altered use. Pflugers Arch. 2008;456(3):587–600.
Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev. 2000;80(2):853–924.
Devries MC, Tarnopolsky MA. Muscle physiology in healthy men and women and those with metabolic myopathies. Neurol Clin. 2008;26(1):115–48.
Carmignac V, Durbeej M. Cell-matrix interactions in muscle disease. J Pathol. 2012;226(2):200–18.
Gillies AR, Lieber RL. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve. 2011;44(3):318–31.
Statland JM, Barohn RJ. Muscle channelopathies: the nondystrophic myotonias and periodic paralyses. Continuum (Minneap Minn). 2013;19(6):1598–614.
Kang JS, Krauss RS. Muscle stem cells in development and regenerative myogenesis. Curr Opin Clin Nutr Metab Care. 2010;13(3):243–8.
Buckingham M, Bajard L, Chang T, Daubas P, Hadchouel J, Meilhac S, et al. The formation of skeletal muscle: from somite to limb. J Anat. 2003;202(1):59–68.
Russell AP. The molecular regulation of skeletal muscle mass. Proc Aust Physiol Soc. 2009;40:85–93.
Wu W, Huang R, Wu Q, Li P, Chen J, Li B, et al. The role of Six1 in the genesis of muscle cell and skeletal muscle development. Int J Biol Sci. 2014;10(9):983–9.
Birbrair A, Zhang T, Wang Z-M, Messi ML, Mintz A, Delbono O. Pericytes: multitasking cells in the regeneration of injured, diseased, and aged skeletal muscle. Front Aging Neurosci. 2014;6:245.
Norrby M, Tagerud S. Mitogen-activated protein kinase-activated protein kinase 2 (MK2) in skeletal muscle atrophy and hypertrophy. J Cell Physiol. 2010;223(1):194–201.
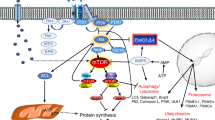
Egerman MA, Glass DJ. Signaling pathways controlling skeletal muscle mass. Crit Rev Biochem Mol Biol. 2014;49(1):59–68.
Sandri M. Signaling in muscle atrophy and hypertrophy. Physiology (Bethesda). 2008;23:160–70.
Gumucio JP, Mendias CL. Atrogin-1, MuRF-1, and sarcopenia. Endocrine. 2013;43(1):12–21.
Gupta VA, Beggs AH. Kelch proteins: emerging roles in skeletal muscle development and diseases. Skelet Muscle. 2014;4:11.
Shadrach JL, Wagers AJ. Stem cells for skeletal muscle repair. Philos Trans R Soc B. 2011;366:2297–306.
Fu X, Wang H, Hu P. Stem cell activation in skeletal muscle regeneration. Cell Mol Life Sci. 2015.
Motohashi N, Asakura A. Muscle satellite cell heterogeneity and self-renewal. Front Cell Dev Biol. 2014;2(1): 00001.
Scharner J, Zammit PS. The muscle satellite cell at 50: the formative years. Skelet Muscle. 2011;1(1):28.
Alway SE, Myers MJ, Mohamed JS. Regulation of satellite cell function in sarcopenia. Front Aging Neurosci. 2014;6:246.
Wang YX, Dumont NA, Rudnicki MA. Muscle stem cells at a glance. J Cell Sci. 2014;127(21):4543–8.
Ma G, Wang Y, Li Y, Cui L, Zhao Y, Zhao B, et al. MiR-206, a key modulator of skeletal muscle development and disease. Int J Biol Sci. 2015;11(3):345–52.
Compliance with Ethics Guidelines
Conflict of Interest
Wladimir Bocca Vieira de Rezende Pinto, Paulo Victor Sgobbi de Souza, and Acary Souza Bulle Oliveira declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Muscle Injuries
Rights and permissions
About this article
Cite this article
de Rezende Pinto, W.B.V., de Souza, P.V.S. & Oliveira, A.S.B. Normal muscle structure, growth, development, and regeneration. Curr Rev Musculoskelet Med 8, 176–181 (2015). https://doi.org/10.1007/s12178-015-9267-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12178-015-9267-x