Abstract
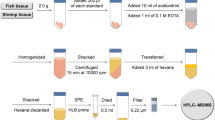
This study reports an analytical method to simultaneously determine the residues of sulfonamides, dapsone, ormethoprim, and trimethoprim in fish and shrimp. The samples were extracted with acetonitrile, magnesium sulfate, and sodium acetate, followed by dispersive solid-phase extraction (C18) for cleanup. The target analytes were confirmed and quantified by liquid chromatography–tandem mass spectrometry. The developed method was validated according to the Codex guidelines (CAC/GL 71-2009). As a result, linearity was expressed between 0.25 and 200 μg kg−1 with a correlation coefficient > 0.98. The limits of quantitation were 0.01–2.8 μg kg−1 and the accuracy (expressed as average recovery) was 76.1–115%. The precision (expressed as the coefficient of variation) was <20%. The decision limits of sulfonamides, dapsone, ormethoprim, and trimethoprim were 94.2–114, 1.3–1.5, 105–111, and 53.3–54 μg kg−1 and detection capabilities of 102–129, 1.4–1.6, 109–120, and 55.6–58.2 μg kg−1, respectively. The proposed method was applied to the analysis of real samples (n = 54) obtained from domestic markets in Korea, resulting in a detection rate of 13% (seven samples). This method is applicable for the efficient determination of sulfonamides, dapsone, ormethoprim, and trimethoprim residues in fish and shrimp according to Korean MRL.



Similar content being viewed by others
References
Baran W, Adamek E, Ziemiańska J, Sobczak A (2011) Effects of the presence of sulfonamides in the environment and their influence on human health. J Hazard Mater 196:1–15. https://doi.org/10.1016/j.jhazmat.2011.08.082
CAC Codex Alimentarius Commission (2015) 38th session of the Codex veterinary drug residue in food online database. Retrieved from http://www.fao.org/fao-who-codexalimentarius/standards/veterinary-drugs-mrls/en/
CAC Codex Alimentarius Commission (2017) Maximum residue limits (MRLs) and risk management recommendations (RMRs) for residues of veterinary drugs in foods. CAC, Rome, Italy, CAC/MRL:2–2017
Cai Z, Zhang Y, Pan H, Tie X, Ren Y (2008) Simultaneous determination of 24 sulfonamide residues in meat by ultra-performance liquid chromatography tandem mass spectrometry. J Chromatogr A 1200:144–155. https://doi.org/10.1016/j.chroma.2008.05.095
CCRVDF Codex Committee on Residues of Veterinary Drugs in Foods (2012) Guidelines for the design and implementation of national regulatory food safety assurance programme associated with the use of veterinary drugs in food producing animals (CAC/GL 71–2009). Codex Alimentarius Commission (CAC), Rome, pp 1–30
Cho SK, Abd El-Aty AM, Park KH, Park J-H, Assayed ME, Jeong Y-M, Park Y-S, Shim J-H (2013) Simple multiresidue extraction method for the determination of fungicides and plant growth regulator in bean sprouts using low temperature partitioning and tandem mass spectrometry. Food Chem 136:1414–1420. https://doi.org/10.1016/j.foodchem.2012.09.068
Dasenaki ME, Thomaidis NS (2015) Multi-residue determination of 115 veterinary drugs and pharmaceutical residues in milk powder, butter, fish tissue and eggs using liquid chromatography–tandem mass spectrometry. Anal Chim Acta 880:103–121. https://doi.org/10.1016/j.aca.2015.04.013
EC European Commission (2002) Council directive 2002/657/EC of 12 August 2002 implementing council directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off J Eur Communities L221:8–36
EC European Commission (2009a) On pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin (Reg. EC no. 37/2010). Off J Eur Comm L15:1–72
EC European Commission (2009b) Council regulation (EEC) no. 470/2009 of 6 May 2009 laying down community procedures for the establishment of residue limits of pharmacologically active substances in foodstuffs of animal origin, repealing council regulation (EEC) no. 2377/90. Off J Eur Comm L152:11–22
Economou A, Petraki O, Tsipi D, Botitsi E (2012) Determination of a liquid chromatography–tandem mass spectrometry method for the determination of sulfonamides, trimethoprim and dapsone in honey and validation according to commission decision 2002/657/EC for banned compounds. Talanta 97:32–41. https://doi.org/10.1016/j.talanta.2012.03.058
Freitas A, Leston S, Rosa J, Castilho MC, Barbosa J, Rema P, Pardal MA, Ramos F (2014) Multi-residue and multi-class determination of antibiotics in gilthead sea bream (Sparus aurata) by ultra high-performance liquid chromatography-tandem mass spectrometry. Food Addit Contam A 31:817–826. https://doi.org/10.1080/19440049.2014.891764
Gibbs RS, Murray SL, Watson LV, Nielsen BP, Potter RA, Murphy CJ (2018) Development and validation of a hybrid screening and quantitative method for the analysis of eight classes of therapeutants in aquaculture products by liquid chromatography–tandem mass spectrometry. J Agric Food Chem 66:4997–5008. https://doi.org/10.1021/acs.jafc.7b05357
Hewavitharana AK (2011) Matrix matching in liquid chromatography–mass spectrometry with stable isotope labelled internal standards—is it necessary? J Chromatogr A 1218:359–361. https://doi.org/10.1016/j.chroma.2010.11.047
Hoff RB, Pizzolato TM, Peralba MCR, Díaz-Cruz MS, Barceló D (2015) Determination of sulfonamide antibiotics and metabolites in liver, muscle and kidney samples by pressurized liquid extraction or ultrasound-assisted extraction followed by liquid chromatography–quadrupole linear ion trap-tandem mass spectrometry (HPLC–QqLIT-MS/MS). Talanta 134:768–778. https://doi.org/10.1016/j.talanta.2014.10.045
Hou X, Lei S, Qiu S, Guo L, Yi S, Liu W (2014) A multi-residue method for the determination of pesticides in tea using multi-walled carbon nanotubes as a dispersive solid phase extraction absorbent. Food Chem 153:121–129. https://doi.org/10.1016/j.foodchem.2013.12.031
Jia W, Shi L, Chu X (2018) Untargeted screening of sulfonamides and their metabolites in salmon using liquid chromatography coupled to quadrupole Orbitrap mass spectrometry. Food Chem 239:427–433. https://doi.org/10.1016/j.foodchem.2017.06.143
Kang H-S, Lee S-B, Shin D, Jeong J, Hong J-H, Rhee G-S (2018) Occurrence of veterinary drug residues in farmed fishery products in South Korea. Food Control 85:57–65. https://doi.org/10.1016/j.foodcont.2017.09.019
Kang H-S, Han S, Cho B-H, Lee H (2019a) Risk-based approach to develop a national residue program: prioritizing the residue control of veterinary drugs in fishery products. Fish Aquat Sci 22:29. https://doi.org/10.1186/s41240-019-0143-2
Kang H-S, Kwon NJ, Jeong J, Lee K, Lee H (2019b) Web-based Korean maximum residue limit evaluation tools: an applied example of maximum residue limit evaluation for trichlorfon in fishery products. Environ Sci Pollut Res 26:7284–7299. https://doi.org/10.1007/s11356-019-04314-y
Kim K, Park D, Kang G, Kim T, Yang Y, Moon S, Choi E, Ha D, Kim E, Cho B (2016) Simultaneous determination of plant growth regulator and pesticides in bean sprouts by liquid chromatography–tandem mass spectrometry. Food Chem 208:239–244. https://doi.org/10.1016/j.foodchem.2016.04.002
Kim J, Park H, Kang H-S, Cho B-H, Oh J-H (2020) Comparison of sample preparation and determination of 60 veterinary drug residues in flatfish using liquid chromatography-tandem mass spectrometry. Molecules 25:1206. https://doi.org/10.3390/molecules25051206
Li J, Liu H, Zhang J, Liu Y, Wu L (2016) A novelty strategy for the fast analysis of sulfonamide antibiotics in fish tissue using magnetic separation with high-performance liquid chromatography–tandem mass spectrometry. Biomed Chromatogr 30:1331–1337. https://doi.org/10.1002/bmc.3693
Liu S, Du J, Chen J, Zhao H (2014) Determination of 19 antibiotic and 2 sulfonamide metabolite residues in wild fish muscle in mariculture areas of Laizhou Bay using accelerated solvent extraction and high performance liquid chromatography-tandem mass spectrometry. Chin J Chromatogr 32:1320–1325. https://doi.org/10.3724/sp.j.1123.2014.08032
Lopes RP, Reyes RC, Romero-González R, Martínez Vidal JL, Frenich AG (2012) Multiresidue determination of veterinary drugs in aquaculture fish samples by ultra high performance liquid chromatography coupled to tandem mass spectrometry. J Chromatogr B 895–896:39–47. https://doi.org/10.1016/j.jchromb.2012.03.011
McDonald M, Mannion C, Rafter P (2009) A confirmatory method for the simultaneous extraction, separation, identification and quantification of tetracycline, sulphonamide, trimethoprim and dapsone residues in muscle by ultra-high-performance liquid chromatography–tandem mass spectrometry according to commission decision 2002/657/EC. J Chromatogr A 1216:8110–8116. https://doi.org/10.1016/j.chroma.2009.05.092
Ministry of Health, Labour and Welfare, Japan (2014) The Japanese positive list system for agricultural chemical residues in foods, Tokyo, Japan. http://www.ffcr.or.jp/ zaidan/FFCRHOME.nsf/pages/MRLs-p. Accessed on March 10, 2015
Nicosia A, Celi M, Vazzana M, Damiano MA, Parrinello N, D’Agostino F, Avellone G, Indelicato S, Mazzola S, Cuttitta A (2014) Profiling the physiological and molecular response to sulfonamidic drug in Procambarus clarkii. Comp Biochem Physiol C Toxicol Pharmacol 166:14–23. https://doi.org/10.1016/j.cbpc.2014.06.006
Nunes KSD, Assalin MR, Vallim JH, Jonsson CM, Queiroz SCN, Reyes FGR (2018) Multiresidue method for quantification of sulfonamides and trimethoprim in tilapia fillet by liquid chromatography coupled to quadrupole time-of-flight mass spectrometry using QuEChERS for sample preparation. J Anal Methods Chem 2018:4506754–4506710. https://doi.org/10.1155/2018/4506754
Park HJ, Kim JH, Kang H-S, Cho B-H, Oh JH (2020) Multi-residue analysis of 18 dye residues in animal products by liquid chromatography-tandem mass spectrometry. J Food Hyg Saf 35:109–117. https://doi.org/10.13103/JFHS.2020.35.2.109
Reeves PT (2012) Antibiotics: groups and properties. Chemical analysis of antibiotic residues in food. Wiley, New Jersey, pp 30–31
SANTE (2015) Guidance document on analytical quality control and method validation procedures for pesticides residues analysis in food and feed European Commission document no SANTE/11945/2015
Serrano PH (2005) Responsible use of antibiotics in aquaculture. FAO, Rome 469
Shin D, Kang H-S, Jeong J, Kim J, Choe WJ, Lee KS, Rhee G-S (2018) Multi-residue determination of veterinary drugs in fishery products using liquid chromatography-tandem mass spectrometry. Food Anal Methods 11:1815–1831. https://doi.org/10.1007/s12161-018-1179-0
Song C, Li L, Zhang C, Kamira B, Qiu L, Fan L, Wu W, Meng S, Hu G, Chen J (2017) Occurrence and human dietary assessment of sulfonamide antibiotics in cultured fish around Tai Lake, China. Environ Sci Pollut Res 24:17493–17499. https://doi.org/10.1007/s11356-017-9442-2
USDA United States Department of Agriculture (2017) Maximum residue limits (MRL) database. Retrieved from https://www.fas.usda.gov/maximum-residue-limits-mrl-database
Zhang K, Wong JW, Yang P, Tech K, DiBenedetto AL, Lee NS, Hayward DG, Makovi CM, Krynitsky AJ, Banerjee K, Jao L, Dasgupta S, Smoker MS, Simonds R, Schreiber A (2011) Multiresidue pesticide analysis of agricultural commodities using acetonitrile salt-out extraction, dispersive solid-phase sample clean-up, and high-performance liquid chromatography–tandem mass spectrometry. J Agric Food Chem 59:7636–7646. https://doi.org/10.1021/jf2010723
Zhang Z, Li X, Ding S, Jiang H, Shen J, Xia X (2016) Multiresidue analysis of sulfonamides, quinolones, and tetracyclines in animal tissues by ultra-high performance liquid chromatography-tandem mass spectrometry. Food Chem 204:252–262. https://doi.org/10.1016/j.foodchem.2016.02.142
Zhu YI, Stiller MJ (2001) Dapsone and sulfones in dermatology: overview and update. J Am Acad Dermatol 45:420–434. https://doi.org/10.1067/mjd.2001.114733
Funding
This study was supported by grants (No. 19161MFDS581 and 20161MFDS623) from the Ministry of Food and Drug Safety in South Korea in 2019 and 2020.
Author information
Authors and Affiliations
Contributions
SYC: performed experimental work and drafted of manuscript
H-SK: study designed and wrote and revised manuscript
Corresponding author
Ethics declarations
Ethical Approval
This article does not contain any studies with human participants or animals by any of the authors.
Informed Consent
Not applicable.
Conflict of Interest
SYC and H-SK declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Choi, S.Y., Kang, HS. Multi-Residue Determination of Sulfonamides, Dapsone, Ormethoprim, and Trimethoprim in Fish and Shrimp Using Dispersive Solid Phase Extraction with LC–MS/MS. Food Anal. Methods 14, 1256–1268 (2021). https://doi.org/10.1007/s12161-021-01965-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-021-01965-x