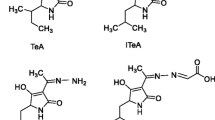
Abstract
An enzyme immunoassay (EIA) was developed based on a generic and highly sensitive monoclonal antibody (mAb) enabling simultaneous detection of all fluoroquinolone/quinolone antibiotics (FQs) approved for the treatment of food-producing animals. To generate the group-specific antibody, norfloxacin was conjugated to glucose oxidase (GlcOX) by one-step carbodiimide method and used as immunogen. For the establishment and optimization of a direct, competitive EIA, three different FQ-horseradish peroxidase (HRP) conjugates were generated. Best results were obtained by using a hapten-heterologous conjugate (clinafloxacin-HRP) prepared by applying a simple and highly efficient periodate method. Under optimized conditions, the assay showed a very high sensitivity, e.g., the detection limit for norfloxacin was 0.02 ng/mL. Furthermore, due to the high affinity of the employed mAb, all FQs approved within the EU for the treatment of food-producing animals can be detected well below their maximum residue levels (MRLs) with IC50 values ranging from 0.16 ng/mL (marbofloxacin) to 3.82 ng/mL (sarafloxacin). Additionally, the established EIA showed cross-reactivity with 23 other FQs and IC50 values ranged from 0.05 ng/mL (rufloxacin) to 73.2 ng/mL (pradofloxacin). Thus, the established EIA could be potentially applied both for the detection of approved and illegally used quinolones in food. To demonstrate the applicability of the assay, artificially contaminated milk, meat, fish, and shrimp samples were analyzed; mean recovery rates were 88.9%, 76.2%, 74.4%, and 77.9%, respectively. These data demonstrate that the developed EIA is suited for the rapid and sensitive monitoring of FQs residues in animal-derived food.



Similar content being viewed by others
Abbreviations
- DMF:
-
dimethylformamide
- EDC:
-
N-ethyl-N′-(3-dimethylaminopropyl)carbodiimide
- EIA:
-
Enzyme immunoassay
- EU:
-
European Union
- GlcOX:
-
Glucose oxidase
- FQs:
-
Fluoroquinolones/quinolones
- HRP:
-
Horseradish peroxidase
- LOD:
-
Limit of detection
- mAb:
-
Monoclonal antibody
- MRL:
-
Maximum residue limit
- PBS:
-
Phosphate buffered saline
- IFA:
-
Incomplete Freund’s adjuvants
- TMB:
-
3,3′,5,5′-tetramethyl-benzidine
- BES:
-
Besifloxacin
- CIN:
-
Cinoxacin
- CIP:
-
Ciprofloxacin
- CLN:
-
Clinafloxacin
- DAN:
-
Danofloxacin
- DEL:
-
Delafloxacin
- DIF:
-
Difloxacin
- ENO:
-
Enoxacin
- ENR:
-
Enrofloxacin
- FLR:
-
Fleroxacin
- FLU:
-
Flumequine
- GAT:
-
Gatifloxacin
- IBA:
-
Ibafloxacin
- LEV:
-
Levofloxacin
- LOM:
-
Lomefloxacin
- MAR:
-
Marbofloxacin
- MOX:
-
Moxifloxacin
- NAD:
-
Nadifloxacin
- NA:
-
Nalidixic acid
- NOR:
-
Norfloxacin
- OFL:
-
Ofloxacin
- ORB:
-
Orbifloxacin
- OA:
-
Oxolinic acid
- PRA:
-
Pradofloxacin
- PAZ:
-
Pazufloxacin
- PEF:
-
Pefloxacin
- PA:
-
Pipemidic acid
- PRU:
-
Prulifloxacin
- RUF:
-
Rufloxacin
- SAR:
-
Sarafloxacin
- SPR:
-
Sparfloxacin
- TOS:
-
Tosufloxacin
- TRO:
-
Trovafloxacin
References
Bremus A, Dietrich R, Dettmar L, Usleber E, Märtlbauer E (2012) A broadly applicable approach to prepare monoclonal anti-cephalosporin antibodies for immunochemical residue determination in milk. Anal Bioanal Chem 403:503–515. https://doi.org/10.1007/s00216-012-5750-z
Bucknall S, Silverlight J, Coldham N, Thorne L, Jackman R (2003) Antibodies to the quinolones and fluoroquinolones for the development of generic and specific immunoassays for detection of these residues in animal products. Food Addit Contam 20:221–228. https://doi.org/10.1080/0265203021000055388
Burkin MA (2008) Enzyme-linked immunosorbent assays of fluoroquinolones with selective and group specificities. Food Agric Immunol 19:131–140. https://doi.org/10.1080/09540100802098331
Cao L, Sui J, Kong D, Li Z, Lin H (2011a) Generic immunoassay of quinolones: production and characterization of anti-pefloxacin antibodies as broad selective receptors. Food Anal Methods 4:517–524. https://doi.org/10.1007/s12161-011-9196-2
Cao Z, Lu S, Liu J et al (2011b) Preparation of anti-lomefloxacin antibody and development of an indirect competitive enzyme-linked immunosorbent assay for detection of lomefloxacin residue in milk. Anal Lett 44:1100–1113. https://doi.org/10.1080/00032719.2010.511733
Chen J, Lu N, Shen X, Tang Q, Zhang C, Xu J, Sun Y, Huang XA, Xu Z, Lei H (2016) Investigation of an immunoassay with broad specificity to quinolone drugs by genetic algorithm with linear assignment of hypermolecular alignment of data sets and advanced quantitative structure-activity relationship analysis. J Agric Food Chem 64:2772–2779. https://doi.org/10.1021/acs.jafc.6b00039
Cheng G, Hao H, Dai M et al (2013) Antibacterial action of quinolones: from target to network. Eur J Med Chem 66:555–562
E.U. (2010) Commission regulation on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. In vol. 37/2010 (pp. 1–72). Retrieved from http://ec.europa.eu/health/files/eudralex/vol-5/reg_2010_37/r
Fan G, Yang R, Jiang J, Chang XY, Chen JJ, Qi YH, Wu SX, Yang XF (2012) Development of a class-specific polyclonal antibody-based indirect competitive ELISA for detecting fluoroquinolone residues in milk. J Zhejiang Univ Sci B 13:545–554. https://doi.org/10.1631/jzus.B1200001
Gosling RJ, Clouting CS, Randall LP, Horton RA, Davies RH (2012) Ciprofloxacin resistance in E. coli isolated from turkeys in Great Britain. Avian Pathol 41:83–89. https://doi.org/10.1080/03079457.2011.640659
Hack R, Märtlbauer E, Terplan G (1987) A monoclonal antibody to the trichothecene T-2 toxin: screening for the antibody by a direct enzyme immunoassay. J Veterinary Med Ser B 34:538–544. https://doi.org/10.1111/j.1439-0450.1987.tb00430.x
He X, Wang GN, Yang K, Liu HZ, Wu XJ, Wang JP (2017) Magnetic graphene dispersive solid phase extraction combining high performance liquid chromatography for determination of fluoroquinolones in foods. Food Chem 221:1226–1231. https://doi.org/10.1016/j.foodchem.2016.11.035
Holtzapple CK, Buckley SA, Stanker LH (1997) Production and characterization of monoclonal antibodies against sarafloxacin and cross-reactivity studies of related fluoroquinolones. J Agric Food Chem 45:1984–1990. https://doi.org/10.1021/jf960754q
Hu K, Huang X, Jiang Y et al (2010) Monoclonal antibody based enzyme-linked immunosorbent assay for the specific detection of ciprofloxacin and enrofloxacin residues in fishery products. Aquaculture 310:8–12. https://doi.org/10.1016/j.aquaculture.2010.08.008
Huet A-C, Charlier C, Tittlemier SA, Singh G, Benrejeb S, Delahaut P (2006) Simultaneous determination of (fluoro) quinolone antibiotics in kidney, marine products, eggs, and muscle by enzyme-linked immunosorbent assay (ELISA). J Agric Food Chem 54:2822–2827
Janecko N, Pokludova L, Blahova J et al (2016) Implications of fluoroquinolone contamination for the aquatic environment—a review. Environ Toxicol Chem 35:2647–2656. https://doi.org/10.1002/etc.3552
Jiang J, Zhang H, Liu J et al (2011a) Development and optimization of an indirect competitive ELISA for detection of norfloxacin residue in chicken liver. Procedia Environ Sci 8:128–133. https://doi.org/10.1016/j.proenv.2011.10.021
Jiang J, Zhang H, Qi Y et al (2011b) Production and characterization of monoclonal antibodies against norfloxacin. Procedia Environ Sci 8:529–535. https://doi.org/10.1016/j.proenv.2011.10.082
Junjie C, Ping C, Jinqing J (2011) Production and characterization of monoclonal antibodies against marbofloxacin. In: International Conference on Human Health and Biomedical Engineering 19–22. 08. 2011, Institute of Electrical and Electronics Engineers Xplore Digital Library. pp 1204–1207
Kato M, Ihara Y, Nakata E et al (2007) Development of enrofloxacin ELISA using a monoclonal antibody tolerating an organic solvent with broad cross-reactivity to other newquinolones. Food Agric Immunol 18:179–187. https://doi.org/10.1080/09540100701763365
Li Y, Ji B, Chen W et al (2008) Production of new class-specific polyclonal antibody for determination of fluoroquinolones antibiotics by indirect competitive ELISA. Food Agric Immunol 19:251–264. https://doi.org/10.1080/09540100802471538
Liu Z, Lu S, Zhao C et al (2009) Preparation of anti-danofloxacin antibody and development of an indirect competitive enzyme-linked immunosorbent assay for detection of danofloxacin residue in chicken liver. J Sci Food Agric 89:1115–1121. https://doi.org/10.1002/jsfa.3514
Liu YZ, Zhao GX, Liu J, Zhang HC, Wang P, Wang JP (2013a) Synthesis of novel haptens against ciprofloxacin and production of generic monoclonal antibodies for immunoscreening of fluoroquinolones in meat. J Sci Food Agric 93:1370–1377. https://doi.org/10.1002/jsfa.5901
Liu YZ, Zhao GX, Wang P et al (2013b) Production of the broad specific monoclonal antibody against sarafloxacin for rapid immunoscreening of 12 fluoroquinolones in meat. J Environ Sci Heal - Part B Pestic Food Contam Agric Wastes 48:139–146. https://doi.org/10.1080/03601234.2013.727668
Liu C, Feng X, Qian H, Fang G, Wang S (2015) Determination of norfloxacin in food by capillary electrophoresis immunoassay with laser-induced fluorescence detector. Food Anal Methods 8:596–603. https://doi.org/10.1007/s12161-014-9936-1
Lombardo-Agüí M, García-Campaña AM, Gámiz-Gracia L, Cruces-Blanco C (2012) Determination of quinolones of veterinary use in bee products by ultra-high performance liquid chromatography-tandem mass spectrometry using a QuEChERS extraction procedure. Talanta 93:193–199. https://doi.org/10.1016/j.talanta.2012.02.011
Lu S, Zhang Y, Liu J, Zhao C, Liu W, Xi R (2006) Preparation of anti-pefloxacin antibody and development of an indirect competitive enzyme-linked immunosorbent assay for detection of pefloxacin residue in chicken liver. J Agric Food Chem 54:6995–7000. https://doi.org/10.1021/jf061309q
Medalla F, Sjölund-Karlsson M, Shin S, Harvey E, Joyce K, Theobald L, Nygren BN, Pecic G, Gay K, Austin J, Stuart A, Blanton E, Mintz ED, Whichard JM, Barzilay EJ (2011) Ciprofloxacin-resistant salmonella Enterica serotype Typhi, United States, 1999-2008. Emerg Infect Dis 17:1095–1098. https://doi.org/10.3201/eid1706.100594
Naeem A, Badshah SL, Muska M et al (2016) The current case of quinolones: synthetic approaches and antibacterial activity. Molecules 21. https://doi.org/10.3390/molecules21040268
Peng D, Wang Y, Feng L, Cao G, Tao Y, Liu Z, Yuan Z (2016) Preparation of broadly specific monoclonal antibodies for simultaneous determination of fluoroquinolone residues in eggs. Food Anal Methods 9:3520–3531. https://doi.org/10.1007/s12161-016-0546-y
Pinacho DG, Sánchez-Baeza F, Marco MP (2012) Molecular modeling assisted hapten design to produce broad selectivity antibodies for fluoroquinolone antibiotics. Anal Chem 84:4527–4534. https://doi.org/10.1021/ac300263m
Rodríguez Cáceres MI, Guiberteau Cabanillas A, Galeano Díaz T, Martínez Cañas MA (2010) Simultaneous determination of quinolones for veterinary use by high-performance liquid chromatography with electrochemical detection. J Chromatogr B Anal Technol Biomed Life Sci 878:398–402. https://doi.org/10.1016/j.jchromb.2009.12.012
Sheng W, Xia X, Wei K, Li J, Li QX, Xu T (2009a) Determination of marbofloxacin residues in beef and pork with an enzyme-linked immunosorbent assay. J Agric Food Chem 57:5971–5975. https://doi.org/10.1021/jf900940n
Sheng W, Xu T, Ma H et al (2009b) Development of an indirect competitive enzyme-linked immunosorbent assay for detection of danofloxacin residues in beef, chicken and pork meats. Food Agric Immunol 20:35–47. https://doi.org/10.1080/09540100802657581
Sheng W, Li Y, Xu X, Yuan M, Wang S (2011) Enzyme-linked immunosorbent assay and colloidal gold-based immunochromatographic assay for several (fluoro)quinolones in milk. Microchim Acta 173:307–316. https://doi.org/10.1007/s00604-011-0560-0
Smith JL, Fratamico PM (2010) Fluoroquinolone resistance in campylobacter. J Food Prot 73:1141–1152. https://doi.org/10.4315/0362-028X-73.6.1141
Strasser A, Dietrich R, Usleber E, Märtlbauer E (2003) Immunochemical rapid test for multiresidue analysis of antimicrobial drugs in milk using monoclonal antibodies and hapten-glucose oxidase conjugates. Anal Chim Acta 495:11–19. https://doi.org/10.1016/j.aca.2003.08.024
Suryoprabowo S, Liu L, Peng J, Kuang H, Xu C (2014) Development of a broad specific monoclonal antibody for fluoroquinolone analysis. Food Anal Methods 7:2163–2168. https://doi.org/10.1007/s12161-014-9863-1
Takeda N, Gotoh M, Matsuoka T (2011) Rapid screening method for quinolone residues in livestock and fishery products using immobilised metal chelate affinity chromatographic clean-up and liquid chromatographyfluorescence detection. Food Addit Contam - Part A Chem Anal Control Expo Risk Assess 28:1168–1174. https://doi.org/10.1080/19440049.2011.587028
Tittlemier SA, Gelinas J-M, Dufresne G et al (2008) Development of a direct competitive enzyme-linked Immunosorbent assay for the detection of fluoroquinolone residues in shrimp. Food Anal Methods 1:28–35. https://doi.org/10.1007/s12161-007-9004-1
Wang Z, Zhu Y, Ding S, He F, Beier RC, Li J, Jiang H, Feng C, Wan Y, Zhang S, Kai Z, Yang X, Shen J (2007) Development of a monoclonal antibody-based broad-specificity ELISA for fluoroquinolone antibiotics in foods and molecular modeling studies of cross-reactive compounds. Anal Chem 79:4471–4483. https://doi.org/10.1021/ac070064t
Wang Y, Shen Y, Xu Z et al (2010) Production and identification of monoclonal antibody against flumequine and development of indirect competitive enzyme-linked immunosorbent assay. Chin J Anal Chem 38:313–317. https://doi.org/10.1016/S1872-2040(09)60029-3
Wen K, Nölke G, Schillberg S, Wang Z, Zhang S, Wu C, Jiang H, Meng H, Shen J (2012) Improved fluoroquinolone detection in ELISA through engineering of a broad-specific single-chain variable fragment binding simultaneously to 20 fluoroquinolones. Anal Bioanal Chem 403:2771–2783. https://doi.org/10.1007/s00216-012-6062-z
Xinyao C, Jinqing J, Zhixing A, et al (2011) Development of monoclonal immunoassays for the determination of lomefloxacin residue. In: International Symposium on the Water Resource and Environmental Protection, Institute of Electrical and Electronics Engineers Xplore Digital Library. pp 2891–2895
Xu X, Liu L, Jia Z, Shu Y (2015) Determination of enrofloxacin and ciprofloxacin in foods of animal origin by capillary electrophoresis with field amplified sample stacking-sweeping technique. Food Chem 176:219–225. https://doi.org/10.1016/j.foodchem.2014.12.054
Zhao C, Liu W, Ling H, Lu S, Zhang Y, Liu J, Xi R (2007) Preparation of anti-gatifloxacin antibody and development of an indirect competitive enzyme-linked immunosorbent assay for the detection of gatifloxacin residue in milk. J Agric Food Chem 55:6879–6884. https://doi.org/10.1021/jf070978g
Acknowledgments
We thank Ms. Brunhilde Minich and Mr. Mostefa Djeffal for the excellent technical support. This study was summarized from the doctoral thesis of Ulas Acaroz.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Ulas Acaroz declares that he has no conflict of interest. Richard Dietrich declares that he has no conflict of interest. Maria Knauer declares that she has no conflict of interest. Erwin Märtlbauer declares that he has no conflict of interest.
Ethical Approval
All institutional and national guidelines for the care and use of laboratory animals were followed.
Informed Consent
Not applicable
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Maximum residue limits (MRLs) for fluoroquinolone/quinolone (FQ) antibiotics in food of animal origin, in accordance with Regulation (EU) 37/2010; The summary of generic immunoassays for the detection of FQs; Schematic representation of the immunogen synthesis via carbodiimide method; UV spectra of Norfloxacin-GlcOX and Clinafloxacin-HRP conjugates with unconjugated starting materials; Standard curves of other FQs used in human medicine or veterinary medicine; Comparative presentation of standard curves for danofloxacin and marbofloxacin in PBS or reconstituted skimmed milk powder; Characteristics of quinolone standard curves generated in reconstituted milk powder which are employed for the recovery analysis; Characteristics of quinolone standard curves generated in PBS which are employed for the recovery analysis of beef, salmon and shrimp samples.
ESM 1
(DOCX 597 kb)
Rights and permissions
About this article
Cite this article
Acaroz, U., Dietrich, R., Knauer, M. et al. Development of a Generic Enzyme-Immunoassay for the Detection of Fluoro(quinolone)-Residues in Foodstuffs Based on a Highly Sensitive Monoclonal Antibody. Food Anal. Methods 13, 780–792 (2020). https://doi.org/10.1007/s12161-019-01695-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-019-01695-1