Abstract
Oily seed extracts were used as research samples in Analytical Laboratory of Raw Materials and Plant Products. Supercritical carbon dioxide extraction on an industrial scale was used to prepare extracts from raw plant materials, usually berry seeds: blackcurrant, raspberry, strawberry, and chokeberry. Gas chromatography analysis was performed on Agilent equipment with single quadrupole mass spectrometer detector and split/splitless injector. Fatty acids (FAs) were determined using indirect method, where they were converted to corresponding methyl esters (FAMEs) during trimethylsulfonium hydroxide solution reaction. Comparing the mass spectrum and retention time peak, more than 30 compounds in different oily extracts were qualitatively identified. Quantification of individual fatty acids was based on two different methods. Firstly, it was based on the received peak area, and the results were normalized without correction factor. Secondly, the quantification was based on external calibration curve, for 34 identified fatty acids. The method was validated, and the results, e.g., linearity, precision, limit of detection (LOD), and limit of quantification (LOQ), were presented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants are not easy to research. They have complicated matrix, which is problematic during analysis (matrix effect) (Yaroshenko and Kartsova 2014); however, they also contain many valuable, healthy substances known as bioactive compounds including flavonoids (polyphenols), phenolic and hydroxycinnamic acids, lignans, vitamins, carotenoids, monoterpenes, and lipids (phytosterols, tocopherols, and saturated and unsaturated fatty acids).
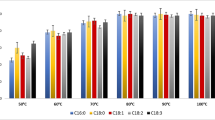
Analysts usually use all parts of plants (roots, stalks, leaves, flowers, fruits, and seeds), as well as juices, concentrates, and extracts which are the final products of the technological processes. Agricultural production and the berry fruit industry have a significant position in Poland (Nawirska et al. 2007; Agricultural Market Agency 2014; Kraciński 2014). In 2013, the fruit share in the value of commodity crop production was more than 15%. In 2004–2013, berry fruit collection, in Poland (Fig. 1), were 5–11% of the fruit products in EU. In 2013, they were at the record level of 4.13 million tons, about 29% higher than average yields in 2004–2012 (Agricultural Market Agency 2014; Kraciński 2014).
The structure of berry fruit crops in Poland in 2013 (Agricultural Market Agency 2014)
What fuelled the growth of fruit production were farmlands in quite good conditions and a large group of well-educated and experienced young fruit growers, especially in the berry fruit sector, which in 2013 was at the level of 607,000 t.
A proper processing base is one of the key factors in the development of berry fruit production. Among the processed fruits, frozen ones dominate (40%), but concentrated juices (30%) and pomaces (16%) also have a significant share.
Extracts can be obtained from pomaces, which are the wastes or by-products in plant production processes and contain up to 50% (w/w) of seeds, rich in nutrients and biological active substances. Dry seeds from blackcurrant, raspberry, or strawberry may contain up to 25% of fat and 20% of proteins, and in this way, they can be an interesting material to receive oily extracts rich in unsaturated and polyunsaturated fatty acids (Nawirska 2007; Rój et al. 2009; Nowak 2005; Dobrzyńska et al. 2014).
There are different methods for obtaining extracts from solid samples like plants and plant seeds. The most popular in laboratories and manufactories were conventional simple solvent extractions (solid–liquid extraction, Soxhlet extraction). Nowadays, liquid–liquid or solid–liquid extractions are usually assisted by external factors (e.g., mechanical agitation, pressing and/or heating system) giving more rapid and automated methods (e.g., pressurized liquid extraction (PLE), ultrasound-assisted extraction (UAE), microwave-assisted extraction (MAE), accelerated solvent extraction (ASE)). They have an advantage over conventional methods because they are time saving and solvent reducing and can be carried out with no oxygen or light which prevents the degradation of desired substances (Nayak et al. 2015; Rombaut et al. 2014; Chemat et al. 2015; Da Porto et al. 2009). Another extraction method, old but reactivated now because of their similarity to “the Green Analytical Chemistry,” is the cold-pressed extraction (Armenta et al. 2008; Chemat et al. 2012; Tobiszewski et al. 2009; Płotka et al. 2013; Tobiszewski and Namieśnik 2012; Van Hoed et al. 2011). As we can read in the Codex Alimentarius, “cold pressed fats and oils are edible vegetable fats and oils obtained by mechanical procedures e.g. expelling or pressing, without the application of heat” (Codex Alimmentarius Commission; Obiedzińska and Waszkiewicz-Robak 2012). The obtained extract can only be purified by washing with water, precipitating, filtrating, or centrifuging.
In general, the food industry prefers “green extraction and processing” to ensure safe and high-quality extracts (Nayak et al. 2015; Chemat et al. 2012).
These assumptions fully comply with another type of extraction: supercritical fluid extraction (SFE). This method has been used in the New Chemical Syntheses Institute in Puławy, Poland, since 2000, and was the first one of this type used in Central and Eastern Europe (Skowroński 2005; Skowroński and Mordecka 2001; Rój 2009; Rój and Skowroński 2006a, b). In the middle of 2011, the Institute launched another research and production of a supercritical CO2 (scCO2) extraction plant to extract oils from raw plant materials. It has 2000 t/year processing ability and works in a pressure range 20–53 MPa (Rój et al. 2009; Rój et al. 2013). SFE is solvent and waste-free, is faster than conventional liquid–liquid methods, and with easy parameter control, provides a certain selectivity (Meyer et al. 2012; Aladić et al. 2015).
In a significant number of publications about scCO2 extraction of raw plants or their parts, we can find that several authors described matrix effects (Aladić et al. 2015; Araus et al. 2009; Azmir et al. 2013), and others discuss optimized reaction parameters in the production of plant oils using, for example, response surface methodology (Rój and Skowroński 2006a, b; Azmir et al. 2013; Stamenic et al. 2010; Watros et al. 2013; Da Porto et al. 2012a, b; Ara et al. 2015). Most of them used scCO2 laboratory-scale extraction installation (up to 2 L extraction vessel) to obtain oils from the most popular fruits, vegetables, or herbs, e.g., grapes, olives, and sunflowers (Yang et al. 2011; Rai et al. 2016; Aladić et al. 2015; Milić et al. 2015; Duba and Fiori 2015; Del Valle 2015; Da Porto et al. 2012a, b), but only a few of them used supercritical CO2 industry-scale extraction plant to obtain oils, especially from berry seeds (Rój et al. 2013).
In this study, scCO2 extracts from blackcurrant, strawberry, raspberry, and chokeberry seeds from an industry-scale plant (extraction vessel 2200 L) were used as samples in order to determine fatty acid content. The composition of oils obtained by the described method can be compared with the oils extracted by other techniques and can be used in the future study on the characteristics of berry seed oils according to various contents of unsaturated and polyunsaturated fatty acids. Also, the achieved value of fatty acid (FA) content can help to choose appropriate oils for, e.g., pharmacy, medicine, or food industry.
Most regulations and quality standards in laboratories required validation of analytical methods. The results from method validation can be used to determine the quality, reliability, and consistency of analytical results, which is an integral part of any good analytical practice. To obtain consistent, reliable, and accurate data from analytical measurement, the method of fatty acid analysis in berry seed oils was validated.
Materials and Methods
Chemicals, Standards, and Reference Materials
All chemicals and reagents were of analytical reagent grade. Analytical standards of FAMEs (methyl butyrate; methyl hexanoate, methyl octanoate; methyl decanoate; methyl undecanoate; methyl laurate; methyl tridecanoate; methyl myristate; methyl pentadecanoate; methyl palmitate; methyl palmitoleate; methyl heptadecanoate; methyl cis-10-heptadecenoate; methyl stearate; methyl elaidate; methyl oleate; methyl linolelaidate; methyl linoleate; methyl γ-linolenate; methyl arachidate; methyl linolenate; methyl cis-11-eicosenoate; methyl heneicosanoate; cis-11,14-eicosadienoic acid methyl ester; cis-8,11,14-eicosatrienoic acid methyl ester; methyl behenate; methyl arachidonate; cis-11,14-17-eicosatrienoic acid methyl ester; methyl tricosanoate; methyl all-cis-5,8,11,14,17-eicosapentaenoate; cis-13,16-docosadienoic acid methyl ester; methyl tetracosanoate; methyl cis-15-tetracosenoate; all-cis-4,7,10,13,16,19-mocosahexaenoic acid methyl ester) were from Sigma-Aldrich Co. (St. Louis, MO, USA). Trimethylsulfonium hydroxide (TMSH) solution (∼0.25 M in methanol) for GC derivatization was delivered by Fluka (Sigma-Aldrich Co., St. Louis, MO, USA). Tert-butyl methyl ether (MTBE) 99.8%, sodium chloride, and isooctane were purchased from POCH S.A. (Gliwice, Poland). Boron trifluoride (∼1.3 M in methanol) was from Fluka (Sigma-Aldrich Chemie GmbH, Steinheim, Switzerland). Standard Reference Material 3251 Serenoa repens Extract was from NIST; Extract Reference Material (XRM) Serenoa serrulata (Saw Palmetto) Fruit CDXA-XRM-001 was from ChromaDex.
All standards and materials were stored in appropriate conditions (a fridge or a freezer).
Plant Material
Seeds of different species of blackcurrant, strawberry, raspberry, and chokeberry were purchased from external suppliers, as a by-product in plant production processes. All obtained scCO2 berry seed extract samples were stored in a fridge, under nitrogen atmosphere, in 25–50-mL orange glass bottles.
Fatty Acid Extraction
scCO2 extraction allows extraction of plant oils at temperature above 35 °C and pressure above 20 MPa. Extracts from blackcurrant, strawberry, raspberry, and chokeberry seeds obtained from scCO2 industry-scale installation, using extraction vessel 2200 L, were prepared and delivered to the laboratory by internal suppliers. According to Rój and collaborators (Rój et al. 2009), during the processes some of scCO2 extraction conditions were changed (extraction time and pressure from 28 to 36 MPa), and others were constant (T = 50 °C; CO2 flow = 80 kg/h), so that the mixture of extracts were analyzed. Some samples were collected separately every 15–20 min and were used to define the FA profile during the cycle time process. Seeds were prepared as described in earlier publications (Rój et al. 2013).
FAME Preparation
In the first part of the experiment, two different methods to obtain FAMEs were used. Both of them are normalized and described in the Polish version of the European Standard EN ISO 5509:2000.
A first few extract samples were prepared as follows: About 150 mg of extract from berry seeds and 4 mL of sodium hydroxide methanol solution (0.5 M) were placed into a 50-mL round-bottom flask. The reflux condenser was placed, and the flask was heated for 25 min. Next, 5 mL of boron trifluoride (BF3 ∼ 1.3 M in methanol) was added via the upper end of a reflux condenser and heated for 5 min. Then, 3 mL of isooctane was added into a boiling mixture, the reflux condenser was disconnected, and the mixture was cooled in an ice water bath, as fast as possible. From 20 to 40 mL saturated NaCl solution was added to the mixture, shaked vigorously, and left for phase separation. The isooctane phase (1–2 mL) was placed into an autosampler vial and directed for GC analysis.
The described method was appropriate for the analyzed type of samples. BF3 as acylation reagent for GC derivatization required temperature about 100 °C, so that the method was solvent- and time-consuming. The high number of samples received from industry plant and necessity of online analysis of FA content requires faster method application.
The second method described in EN ISO 5509 was used and validated. Samples (without internal standards) were prepared at room temperature as follows: About 10 mg ± 3 mg of analytical sample was placed into a 1.5-mL autosampler vial. Five hundred microliters of tert-butyl methyl ether was added and mixed for 5 min to dissolve the sample. Next, 250 μL of TMSH solution (∼0.25 M in methanol) was added and, without heating, all mixture was shaken for 25 min. Samples were usually diluted 1:10 or 1:20 with MTBE/CH3OH (9:1 v/v). After that, the sample vial was placed into the autosampler and analyzed.
GC-MSD Analysis
Gas chromatography analysis were performed on Agilent equipment (GC 6890N) with single quadrupole mass spectrometer detector (MSD 5975) and split/splitless injector. Both systems were controlled by MSD ChemStation, version E.02.02.1431 (Agilent Technologies, Inc.). An Agilent J&W GC capillary column, type HP-88, with 88% cyanopropylaryl polysiloxane phase (60 m, 0.25 mm i.d., 0.20 μm film thickness) was used to separate FAMEs. Some notes or applications (EN-ISO 5509 2000; Sigma-Aldrich Brochure 2007) do not recommend this type of column phase with TMSH-prepared samples, especially during cold on-column injection or when lipids with hydroxy groups occur. Any undesirable effects were not observed in our analysis. The oven temperature program was started at 90 °C held for 2 min, increased to 152 °C at a rate of 4 °C/min, held for 1 min, and increased to 218 °C at the rate of 2 °C/min, held for 1 min. Total analysis time was 53 min. The split/splitless injector was used with injector temperature 250 °C and split ratios 6:1 and 120:1. Helium was used as a carrier gas, with a flow rate of 1.5 mL/min. Inject volume was 1 μL, solvent delay 3.5 min, and MSD ionization voltage 70 eV. Data were collected in SCAN and SIM mode. FAMEs were identified by comparing their mass spectrum (Fig. 2) and fragmentation patterns in the NIST library and by comparing retention time peak with appropriate standards. The FA contents were expressed as weight percentages, % w/w (g FA/100 g of sample). Samples were prepared separately in duplicates, and the average values were presented as final results. The obtained data were analyzed statistically during method validation using internally prepared spreadsheets in Microsoft Office Excel 2007 and online available authorized computer program e-Stat (available in Polish version).
Results and Discussion
Chromatography Results
The pro-health approach to fatty acids forced the producers of oils and food, as well as companies that deal with this subject, to check the level and content determination of individual fatty acids and their isomers. It is not easy, especially for little-known matrix, and the extracts of oil obtained by scCO2 extraction are this kind of matrices.
All unsaturated lipids are oxidized under the influence of many external factors, and spontaneous oxidation is observed with regard to mainly unsaturated hydrocarbon chains of fatty acids. The rate of this reaction increases with the increase in the degree of unsaturation. According to the literature, linoleic acid is oxidized 10–40 times faster than oleic acid and linolenic acid 2–4 times faster than linoleic acid (Drozdowski 2007).
The developed method allows the preparation of a draft specification of oil extracts and indicates the quantity and nature of present acids (cis/trans isomers and saturated and unsaturated acids), from which you can determine the durability of the extract and the rate in the oxidation process.
A typical chromatogram of the analysis of the chosen 34-compound FAME standard, obtained on the HP-88 column, is shown in Fig. 3.
A very good separation is obtained, except the following compounds: C20:4 (n6) coelute at 39.5 min with C20:3 (n3). Also very close, but separated, are C20:5 (n3) at 42.1 min and C22:2 at 42.2 min. However, this separation is sufficient for the analyzed extract from the chosen berry seeds.
Using this method, all FAs can by determined in scCO2-obtained extracts. This is demonstrated in Figs. 4 and 5, where the analysis of blackcurrant and raspberry seed oil samples is shown. Peaks are identified by giving retention time presented in Table 1.
Free FAs can be analyzed directly on polar stationary phases, but more robust and reproducible chromatographic data are obtained if the fatty acids are derivatized to the corresponding methyl esters. Different methods and reagents are available for the derivatization, selective response, and detection in complex matrices (Sigma-Aldrich Brochure 2007). The TMSH method, mentioned in this research paper, is easy to use and does not require expensive equipment and reagents. Also, during reaction with TMSH, there is no isomerization of polyunsaturated fatty acids and removing excess of reagent is not required, because in the injector at a temperature of 250 °C pyrolysis occurs (methanol and (CH3)2S evaporate).
Using an HP-88 column and the presented gradient temperature program, all compounds in the standard mixture and in the berry seed extracts are well separated. What is important is the separation of cis/trans isomers like C18:1 (n9) cis/trans and C18:2 (n6) cis/trans and the separation of polyunsaturated components like C20:5 (n3) EPA (42.1 min) and C22:6 (n3) DHA (48.4 min). This method is useful for the determination of omega-3 fatty acids (such as EPA and DHA) and also omega-6 fatty acids, such as C18:2 cis (n6), C18:3 (n6), C20:2 (n6), C20:3 (n6), and C20:4 (n6) presented at retention times 31.8, 33.2, 37.0, 38.5, and 39.5 min, respectively. Figure 4 or 5 demonstrates that for real samples containing several acids and different isomers, separation with the HP-88 column is a good choice.
Examining the qualitative composition of selected samples presented in Table 1 and changes to the composition, during the extraction process (Table 2, Fig. 6), it can be concluded that these extracts obtained a good source of unsaturated fatty acids.
For the analyzed samples of oily extracts, more than 30 fatty acids were shown. The basic ten are C8:0, C10:0, C12:0, C14:0, C16:0, C18:0, C18:1 cis (n9), C18:2 cis (n6), C18:3 (n6), and C18:3 (n3). The others are present in the amounts of less than 0.2% or below specified values of detection limits of the analytical method.
The highest total content of fatty acids was obtained during the analysis of the most easily accessible material of blackcurrant seeds. Total content of FAs was 63–78%, and in one sample even 88%. Next comes a strawberry extract with values of the total content of fatty acids from 50 to 69%, the raspberry interval 44–66%, and chokeberry 25–58%. As shown in Table 1, one raspberry sample had a content of total FAs of 98%. The biggest change in the composition of the extract relates to changes in extraction time. Changes in pressure did not affect significantly the profile change of fatty acids and their concentrations (Dobrzyńska et al. 2014).
Among the saturated fatty acids, palmitic acid dominates, whose value ranges from 3.2% w/w to 9.2% w/w for blackcurrant extract (average concentration about 5.2% w/w in analyzed samples), 0.9–4,5% w/w for raspberry (average conc. 3.5% w/w), 1.4–3.5% w/w for chokeberry (average conc. 3.3% w/w), and 1.7–3.2% w/w for the extract of strawberry seeds (average conc. 2.8% w/w). Also, stearic acid is present and berry seed oils contain it in the amount of 1.3% w/w for blackcurrant and 0.7–0.9% w/w for raspberry and strawberry oils, and 0.6% w/w average content was found in the samples of chokeberry.
In the studied samples, there is high content of unsaturated fatty acids (UFA). They accounted for over 90% of the total FAs in received extracts (UFA/total FAs: 94.6% for raspberry, 93.5% for strawberry, 92.6% for chokeberry, 91.3% for blackcurrant). From UFA, three of the fatty acids (C18:2 cis (n6), C18:3 (n3), C18:3 (n6)) are the measures of good-quality test plant materials. Blackcurrant seed oil contains more than 9.8% w/w of C18:3 (n3). Significantly, higher levels of alfa-linolenic acid (ALA) were obtained for the analyzed raspberry oil (average conc. 22.7% w/w) and strawberry oil (average conc. 18.9% w/w). This high level of ALA, especially in the raspberry, strawberry, and blackcurrant oils, makes them interesting products with a positive n-6/n-3 ratio. It is also interesting when we think that those extracts are obtained from the seeds separated from the pomaces—waste or by-products in plant production processes. Furthermore, blackcurrant seed extracts contain more than 10.9% w/w of C18:3 (n6) gamma-linolenic acid, which is the active component in the n-6 fatty acid group.
Method Validation
A method was validated for the quantitation of fatty acids in scCO2-obtained berry seed extracts using GC/MSD Agilent equipment. As a representative sample of the berry seed oils, blackcurrant oil was selected. The concentration range of target FAs used in this validation was chosen to fit the commonly encountered range of analyte concentration in previously tested samples. Therefore, the standard concentrations of the basic eight fatty acids were at a higher level than the rest of them.
Matrix samples, blanks, standards, and reference materials were used during the validation process. Berry seed oils were tested without internal standards. Samples were analyzed in duplicates. All 34 available standards were prepared separately in volume flasks by dissolving an appropriate weight in 25 mL of MTBE. For linoleic acid methyl ester (C18:2 n6), the weight was 0.50 g giving a concentration of 20.0 mg/mL; for methyl laurate (C12:0), methyl myristate (C14:0), methyl palmitate (C16:0), methyl stearate (C18:0), methyl oleate (C18:1), methyl γ-linolenate (C18:3 n6), and methyl linolenate (C18:3 n3), it was 0.125 g into 25 mL of MTBE giving a concentration of 5.0 mg/mL FAME, and for the rest of the 26 FAMEs, it was 0.025 g/25 mL of MTBE giving the final concentration of 1.0 mg/mL. Next, standards on these base concentrations were diluted 50 times, in one flask, to obtain a mix of FAMEs in concentrations of 0.4, 0.1, and 0.02 mg/mL, respectively. All these concentrations could be converted into weight percentage of FAMEs or FAs and all standard solutions diluted again, if needed.
The analytical method was validated according to English and Polish versions of European standards (PN-ISO 3534-1 2009; PN-ISO 3534-2 2010; PN-ISO 5725-1-6 2002; ISO/TS 21748 2004; PN-EN ISO/IEC 17025 2005), internal test procedures, and standard protocols (IB03 research procedure, the research methods validation; IB05 research procedure, the estimation of measurement uncertainty). It was not validated for robustness, carryover, dilution integrity, or mass spectrometer parameter changes (e.g., ion source and quadruple temperatures, ionization voltage). Validation studies included sensitivity measured by the limit of detection (LOD) and limit of quantitation (LOQ), working range, linearity and calibration model fits (correlation), precision (repeatability expressed by standard deviation and relatively standard deviation), accuracy (recovery pattern, certificated value of CRM), coefficient of variation, and uncertainty. All calculations were performed using the ChemStation software Excel 2007 and online available authorized computer program e-Stat.
Limit of Detection and Limit of Quantitation
Limit of detection (LOD) is the lowest concentration of analyte in the sample that can be detected but not necessarily quantified. The limit of quantification (LOQ) is generally determined by the analysis of samples with known concentrations of analyte and by establishing the minimum level at which the analyte can be quantified with acceptable accuracy and precision.
In this paper, a standard mixture, dissolved in MTBE, was used as the sample because it contained all 34 FAs. The sample extract from berry seeds that contains all those selected to identify acids was not found.
Also, a calibration curve equation was used to assess the LOD and LOQ values.
Samples with decreasing amounts of the analyte were injected. They were prepared by 2-, 5-, 8-, and 10-fold diluting standard mixtures of FAMEs at the concentrations of 0.4, 0.1, and 0.02 mg/mL to final concentrations of 0.04, 0.01, and 0.002 mg/mL. This prepared sample was analyzed 10 times. Average concentration values and standard deviations were calculated. LOD was equal to the sum of the total blank value and three times the value of the standard deviation. LOQ was equal to three times the value of LOD. The identical results of LOD and LOQ for all 34 fatty acids were achieved by performing the calculation based on the equation of the linear calibration curve. LOD was then equal to six times the value of the residual standard deviation (sy/x) divided by the value of the slope (a). LOQ was equal to 10 times the sy/x divided by the value of the slope. All individual values for LOD and LOQ are presented in Table 3. Generally, LOD was 0.03% w/w and LOQ was 0.05% w/w.
Range, Linearity, and Calibration Model Fits
The identification criteria for working range determination (calibration curve range) were LOQ, linearity, and calibration model fits (correlation). The working range was set as the range of concentrations from the LOQ to the maximum of the calibration curve, maintaining the correlation coefficient (r 2) above 0.995.
The calibration curves were constructed over the range of 0.4–0.004 mg/mL by replicate injections (n = 3) of standard mixtures. The calibration curves, determined by the least squares regression method, were linear over the range, with r 2 above 0.995 (see Table 3). It was found that the linear fit was an appropriate calibration model for all 34 fatty acids in the analyzed samples.
Figure 7 shows the example calibration curve for oleic acid (C18:1 cis n9).
Precision
The precision of the samples was measured and evaluated as the repeatability expressed by standard deviation (sd) and relative standard deviation (rsd) and as the coefficient of variance (% CV) for the inter-run analysis. The standard acceptance criteria for inter-run precision were ±30% at each concentration. The inter-run precision, as shown in Table 3, ranged from 5 to 29% CV and was within the acceptance criteria for most of FAs in the blackcurrant extract. Only for eight fatty acids was % CV higher than 30% (values marked in red), but it is worth noticing that seven of them were at the LOQ-level concentration (Table 1).
Accuracy
In accordance with current metrological nomenclature, the accuracy is defined as the closeness of agreement between the true value or an accepted reference value and the value found. The true value can be obtained from an established reference method or by sample analysis with known concentrations, for example certified reference material (CRM), standard reference material (SRM), or extract reference material (XRM).
To assess the accuracy and recovery in our method, materials (NIST SRM 3251 Serenoa repens Extract, ChromaDex Extract Reference Material (XRM) Serenoa serrulata (Saw Palmetto) Fruit CDXA-XRM-001) and spiked extract samples of blackcurrant seeds were used. The sample matrix was spiked with the known standard amount, by volume. The concentration should cover the range of concern and should include the amount close to the LOQ, in the middle of the range and at the high end of the calibration curve. Acceptance criteria for recovery were 100 ± 30%. During this analysis, samples were spiked with concentrations in the middle of the range. Accuracy results are shown in Table 4. The recovery ranges from 78 to 125% for selected analytes. The SRM/XRM accuracy ranged from 71 to 109%.
Table 4 shows all the following validation parameters (for selected FAs):
-
1.
Linear regression: a—slope, b—intercept, sa—standard uncertainty (standard deviation) of slope, sb—standard uncertainty (standard deviation) of intercept, sy/x—residual standard deviation, sm—method standard error, CV—coefficient of variance, r 2—correlation coefficient
-
2.
Statistical significance factors: tcrit—Student t critical value in a two-sided test, ta—slope significance factor, tb—intercept significance factor, tr—correlation significance factor
-
3.
Accuracy (recovery pattern, certificated value of SRM/XRM)
-
4.
Expanded uncertainty
Uncertainty
The experimental approach was used to define uncertainty. Expanded uncertainty U(y) was calculated by Eq. (1), where k is the coverage factor (equal 2 for 95% confidence level) and u(y) is the combined uncertainty.
The values of the expanded uncertainty were determined for certain levels of concentration. The highest value (among selected seven FAs) was 33% for C24:0 (conc. range 0.05–0.10% w/w). For C18:3 (n3) and (n6), the value was 19% (conc. range 0.50–0.75% w/w), 15% for C16:0, C18:2 (n6) (conc. range 0.25–0.36% w/w, 1.82–2.36% w/w), 14% for C18:1 (n9) (conc. range 0.51–0.73% w/w), and 13% for C18:0 (conc. range 0.05–0.09% w/w). Taking into account the value of the expanded uncertainty, the results can be represented by Eq. (2), where cFA is the concentration of fatty acid.
Conclusion
Validation shows that the GC/MSD method provides reliable results for the quantitation of FAs. The method displays good accuracy and precision, as well as recovery and uncertainty.
The developed method, based on existing standards and regulations, allows the initial characterization of selected berry seed scCO2-obtained extracts. Chemical composition characteristics, including the content of fatty acids, which could be carried out using the gas chromatography technique, are an important element connecting the chemical industry with research and allow the optimization of scCO2 extraction processes. The whole procedure allows better quality control of the final product (high-quality polyunsaturated oils), evaluates its suitability for a specific market (medical, pharmaceutical, cosmetic, food), and provides the ability for monitoring and modifying the process parameters.
References
Aladić K, Jarni K, Barbir T, Vidović S, Vladić J, Bilić M, Jokić S (2015) Supercritical CO2 extraction of hemp (Cannabis sativa L.) seed oil. Industry Crops and Products 76:472–478
Ara KM, Jowkarderis M, Raofie F (2015) Optimization of supercritical fluid extraction of essential oils and fatty acids from flixweed (Descurainia Sophia L.) seed using response surface methodology and central composite design. J Food Sci Technol 52:1353. doi:10.1007/s13197-014-1353-3
Araus K, Uquiche E, del Valle JM (2009) Matrix effects in supercritical CO2 extraction of essential oils from plant material. J Food Eng 92:438–447
Armenta S, Garrigues S, de la Guardia M (2008) Green analytical chemistry. Trends Anal Chem 27:497–511. doi:10.1016/j.trac.2008.05.003
Azmir J, Zaidul ISM, Rahman MM, Sharig KM, Mohamed A, Sahena F, Jahurul MHA, Ghefoor K, Norulaini NAN, Omar AKM (2013) Techniques for extraction of bioactive compounds from plant materials: a review. J Food Eng 117:426–436
Chemat F, Vian MA, Cravotto G (2012) Green extraction of natural products. Concept and principles. Int J Mol Sci 13:8615–8627
Chemat F, Fabiano-Tixier AS, Vian MA, Allaf T, Vorobier E (2015) Solvent-free extraction of food and natural products. TrAC Trends Anal Chem. doi:10.1016/j.trac.2015.02.021
Codex Alimmentarius Commission: Section 1. Codex general standard for fats and oils, Codex standard for edible fats and oils not covered by individual standards (CODEX STAN 19-1981, Rev. 2_1999), FAO Corporate Document Repository
Da Porto K, Decorti D, Kikic I (2009) Flavour compounds of Lavandula angustifolia L. to use in food manufacturing: comparison of three different extraction methods. Food Chem 112:1072–1078
Da Porto C, Decori D, Tubaro F (2012a) Fatty acid composition and oxidation stability of hemp (Cannabis sativa L.) seed oil extracted by supercritical carbon dioxide. Ind. Crops Prod 36:401–404
Da Porto C, Voinovich D, Decorti D, Natolino A (2012b) Response surface optimization of hemp seed (Cannabis sativa L.) oil yield and oxidation stability by supercritical carbon dioxide extraction. J Supercrit Fluids 68:45–51
Del Valle JM (2015) Extraction of natural compounds using supercritical CO2: going from the laboratory to the industrial application. J Supercrit Fluids 96:180–199
Dobrzyńska A, Kostrzewa D, Rój E, Grzęda K, Kowalski R (2014) Recovery of oils from domestic plant materials by extraction with supercritical carbon dioxide. Chem Rev. doi:10.12916/przemchem.2014.480
Drozdowski B (2007) Lipids—general characteristics of edible fats. In: Food chemistry saccharides lipids proteins, (ed) Sikorski ZE, 5th edn. WNT, Warsaw, Poland
Duba KS, Fiori L (2015) Supercritical CO2 extraction of grape seed oil: effect of process parameters on the extraction kinetics. J Supercrit Fluids 98:33–43
EN-ISO 5509(2000) Animal and vegetable fats and oils—preparation of methyl esters of fatty acids
Fruit market in Poland (2014) Agricultural Market Agency, http://www.arr.gov.pl/data/00321/rynek_owocow2014_pl.pdf
ISO/TS 21748 (2004) Guide to the use of repeatability, reproducibility and trueness estimates in measurement uncertainty estimation
Kraciński P (2014) Harvesting and disposal of the production of strawberries, raspberries and currants in Poland in 2001–2012. Annals of Agricultural Economics and Rural Development 101:132–140
Meyer F, Jaeger P, Eggers R, Stamenic M, Milovanovic S, Zizovic I (2012) Effect of CO2 pre-treatment on scCO2 extraction of natural material. Chem Eng Process Process Intensif 56:37–45
Milić V, Yusup S, Damjanović V, Chan YH (2015) Kinetic modeling of supercritical carbon dioxide extraction of sage (Salvia officinalis L.) leaves and jatropha (Jatropha curcas L.) seeds. J Supercrit Fluids 100:142–145
Nawirska A (2007) Management of waste provided by fruit–vegetable processing industry. Fruit, Vegetable and Fermentation Industry 51:44–46
Nawirska A, Sokół-Łętowska A, Kucharska AZ (2007) The antioxidant properties of fruit marc selected color. Food Science Technology Quality 4:120–125
Nayak B, Dahmoune F, Moussi K, Remini H, Dairi S, Aonn O, Khodir M (2015) Comparison of microwave, ultrasound and accelerated–assisted solvent extraction for recovery of polyphenols from Citrus sinesis peels. Food Chem 187:507–516
Nowak R (2005) Fatty acids composition in fruits of wild rose species. Acta Soc Bot Pol 74:229–235
Obiedzińska A, Waszkiewicz-Robak B (2012) Cold-pressed oils as a functional food. Food Science Technology Quality 1:27–44
Płotka J, Tobiszewski M, Sulej AM, Kupska M, Górecki T, Namieśnik J (2013) Green chromatography review. J Chromatogr A 1307:1–20
PN-ISO 3534-1(2009) PN-ISO 3534-2:2010 Statistics—vocabulary and symbols, parts 1–2, Polish version
PN-ISO 5725-1-6 (2002) Accuracy (trueness and precision) of measurement methods and results, Parts 1–6, Polish version
PN-EN ISO/IEC 17025 (2005) General requirements for the competence of calibration and testing laboratories
Rai A, Mohanty B, Bhargara R (2016) Supercritical extraction of sunflower oil: a central composite design for extraction variables. Food Chem:647–659
Rombaut N, Tixier A, Bily A, Chemat F (2014) Green extraction processes of natural products as tools for biorefinery. Biofuels Bioproducts and Biorefining 8:530–544
Rój E (2009) Processing of hops using supercritical extraction in Poland. Chemik 62:122–125
Rój E, Dobrzyńska-Inger A, Kostrzewa D, Kołodziejczyk K, Sójka M, Król B, Miszczak A, Markowski J (2009) Preparation of oil extracts from berry seeds using CO2 in supercritical conditions. Chem Rev 88:1325–1330
Rój E, Dobrzyńka-Inger A, Grzęda K, Kostrzewa D (2013) Supercritical extraction of plant materials. Chem Rev 92:1358–1363
Rój E, Skowroński B (2006a) Optimizing the manufacturing costs of hop extracts. Chem Rev 85:655–657
Rój E, Skowroński B (2006b) Modeling hop extraction process in supercritical conditions. Chem Rev 85:1140–1141
Sigma-Aldrich Brochure (2007) Derivatization reagents for selective response and detection in complex matrices
Skowroński B (2005) Process of hop extraction with CO2 in supercritical conditions. Pol J Chem Technol 7:29–32
Skowroński B, Mordecka Z (2001) Polish plant for supercritical extraction of hop. Chem Rev 80:521–523
Stamenic M, Zizovic I, Eggers R, Jaege P, Heinrich H, Rój E, Ivanovic J, Skala D (2010) Swelling of plant material in supercritical carbon dioxide. J Supercrit Fluids 52:125–133
Tobiszewski M, Mechlińska A, Zygmunt B, Namieśnik J (2009) Green analytical chemistry in sample preparation for determination of trace organic pollutants. TrAC Trends Anal Chem 28:943–951. doi:10.1016/j.trac.2009.06.001
Tobiszewski M, Namieśnik J (2012) Direct chromatographic methods in the context of green analytical chemistry. TrAC Trends Anal Chem 35:67–73. doi:10.1016/j/trac.2012.02.006
Van Hoed V, Barbouche I, De Clereq N, Dewettinck K, Slah M, Leber E, Verhe R (2011) Influence of filtering of cold pressed berry seed oils on their antioxidant profile and quality characteristics. Food Chem 127:1848–1855
Watros A, Rój E, Dobrzyńska-Inger A, Mazurek B (2013) Determining the composition of plant extracts using response surface methodology. In: Science and industry—spectroscopic methods in practice, new challenges and opportunities, (ed) Hubicki Z, UMCS, Lublin, Poland, p. 173–176
Yang B, Ahotupa M, Määttä P, Kallio H (2011) Composition and antioxidative activities of supercritical CO2–extracted oils from seeds and soft parts of northern berries. Food Res Int 44:2009–2017
Yaroshenko DV, Kartsova LA (2014) Matrix effect and methods for its elimination in bioanalytical methods using chromatography–mass spectrometry. J Anal Chem 69:31–317. doi:10.1134/S1061193481404133
Acknowledgements
The authors would like to thank the employees of the Supercritical Extraction Department at the New Chemical Syntheses Institute for providing analytical samples of berry seed extracts.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Not applicable.
Conflict of Interest
B. Mazurek declares that she has no conflict of interest. M. Chmiel declares that he has no conflict of interest. B. Górecka declares that she has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Mazurek, B., Chmiel, M. & Górecka, B. Fatty Acids Analysis Using Gas Chromatography-Mass Spectrometer Detector (GC/MSD) - Method Validation Based on Berry Seed Extract Samples. Food Anal. Methods 10, 2868–2880 (2017). https://doi.org/10.1007/s12161-017-0834-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-017-0834-1