Abstract
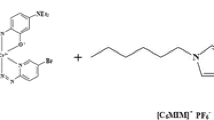
A simple and rapid method based on ultrasound-assisted temperature-controlled ionic liquid microextraction combined with flame atomic absorption spectrometry (FAAS) has been developed for determination of tin. In this method, Sn(IV)-ammonium pyrrolidinedithiocarbamate (APDC) complex was extracted into a small volume of 1-hexyl-3-methylimidazolium hexafluorophosphate as ionic liquid and after phase separation, the enriched analyte in the final solution is determined by FAAS. Experimental design approaches were used to obtain the best conditions. The variables of interest were temperature, pH, buffer volume, extraction time, centrifugation time and volumes of ionic liquid, methanol, and APDC. The Plackett–Burman design was employed for screening to determine the variables significantly affecting the extraction efficiency. Then, the significant factors were optimized by using a central composite design. In the optimal conditions the calibration graph was linear in the range of 0.10–6.0 mg L−1 with a correlation coefficient of 0.997. The limit of detection and relative standard deviation (n = 5 for determination of 1.0 mg L−1) were 42 μg L−1 and 1.6 %, respectively. The preconcentration factor was calculated to be 52.7. Finally, the method was successfully applied to the determination of tin in various canned products including peach, pineapple and aloe vera juice, canned pea, and canned cheese.



Similar content being viewed by others
References
Anderson JRA, Garnett JL, Lock LC (1960) The use of naphthalene derivatives in inorganic analysis: the effect of substituents in the naphthalene nucleus on the fluorimetric detection of tin. Anal Chim Acta 22:1–7
Arya SP, Bansal A (1994) Rapid and selective method for the spectrophotometric determination of tin using potassium ethylxanthate. Microchim Acta 116:63–71
Bermejo-Barrera P, Soto-Ferreiro RM, Bermejo-Barrera A (1993) Direct determination of tin in tap waters by electrothermal atomization atomic absorption spectroscopy. Fresenius J Anal Chem 345:60–62
Blunden S, Wallace T (2003) Tin in canned food: a review and understanding of occurrence and effect. Food Chem Toxic 41:1651–1662
Bordagaray A, Garcia-Arrona R, Millán E (2011) Optimization of solid-phase microextraction procedure coupled to GC-ECD for triazole fungicides determination in juice samples. Food Anal Methods 4:293–299
Bruns RE, Scarminio IS, De Barros NB (2006) Statistical design—chemometrics. Elsevier, Dordrecht, pp 273–277
Capitán-Vallvey L, Valencia M, Mirón G (1994) Flow-injection method for the determination of tin in fruit juices using solid-phase spectrophotometry. Anal Chim Acta 289:365–370
Carrión N, Itriago AM, Alvarez MA, Eljuri E (2003) Simultaneous determination of lead, nickel, tin and copper in aluminium-base alloys using slurry sampling by electrical discharge and multielement ETAAS. Talanta 61:621–632
Codex Alimentarius Commission (2010) Procedural manual, foods and agriculture. Organization of the UN/World Health Organization, Geneva
Costa ACS, Teixeira LSG, Ferreira SLC (1995) Spectrophotometric determination of tin in copper-based alloys using pyrocatechol violet. Talanta 42:1973–1978
Costa HHV, Fátima Lima G, Nacano LR, Tarley CRT (2010) Preconcentration/cleanup studies of tin from environmental water samples by oxidized multiwall carbon nanotubes packed column and its determination by ETAAS. Water Air Soil Pollut 217:557–565
Coyle CF, White CE (1957) Fluorometric determination of tin with flavonol. Anal Chem 29:1486–1488
Fang Z, Sun L, Hansen EH et al (1992) The determination of trace amounts of tin by flow-injection hydride generation atomic-absorption spectrometry with on-line ion-exchange separation and preconcentration. Talanta 39:383–390
Filer TD (1971) Fluorometric determination of submicrogram quantities of tin. Anal Chem 43:1753–1757
Gholivand MB, Babakhanian A, Rafiee E (2008) Determination of Sn(II) and Sn(IV) after mixed micelle-mediated cloud point extraction using α-polyoxometalate as a complexing agent by flame atomic absorption spectrometry. Talanta 76:503–508
Gutierrez AM, Perez-Conde C, Rebollar MP, Diez LMP (1985) A rapid extractive spectrophotometric method for the determination of tin in canned foods with 5,7-dichloro-8-quinolinol. Talanta 32:927–929
Hamdan AJ (2010) Sn (II) selective 2-amino-1,4-naphthoquinone derived poly(vinyl chloride) membrane sensors. Intern J Electrochem Sci 5:215–231
Haug HO, Yiping L (1995) Automated determination of tin by hydride generation using in situ trapping on stable coatings in graphite furnace atomic absorption spectrometry. Spectrochim Acta B 50:1311–1324
Huang X, Zhang W, Han S, Wang X (1997) Determination of tin in canned foods by UV/visible spectrophotometric technique using mixed surfactants. Talanta 44:817–822
ISO 2447 International Organization for Standardization, Geneva, Switzerland (1998)
Khuri AI (2005) Response surface methodology and related topics. World Scientific, Hackensack, pp 123–140
Li YH, Long H, Zhou F-Q (2005) Determination of trace tin by catalytic adsorptive cathodic stripping voltammetry. Anal Chim Acta 554:86–91
Locatelli C, Torsi G (2004) Simultaneous square wave anodic stripping voltammetric determination of Cr, Pb, Sn, Sb, Cu, Zn in presence of reciprocal interference: application to meal matrices. Microchem J 78:175–180
López-García I, Arnau-Jerez I, Campillo N, Hernández-Córdoba M (2004) Determination of tin and titanium in soils, sediments and sludges using electrothermal atomic absorption spectrometry with slurry sample introduction. Talanta 62:413–419
Malla ME, Alvarez MB, Batistoni DA (2002) Evaluation of sorption and desorption characteristics of cadmium, lead and zinc on Amberlite IRC-718 iminodiacetate chelating ion exchanger. Talanta 57:277–287
Manzoori JL, Amjadi M, Abolhasani D (2006) Spectrofluorimetric determination of tin in canned foods. J Hazard Mater 137:1631–1635
Mino Y (2006) Determination of tin in canned foods by X-ray fluorescence spectrometry. J Health Sci 52:67–72
Moreda-Piñeiro J, López-Mahía P, Muniategui-Lorenzo S et al (2002) Tin determination in marine sediment, soil, coal fly ash and coal slurried samples by hydride generation-electrothermal atomic absorption spectrometry. Anal Chim Acta 461:261–271
Moreda-Piñeiro J, Moscoso-Pérez C, Piñeiro-Iglesias M et al (2007) As, Bi, Sb and Sn determination in atmospheric particulate matter by direct solid sampling-hydride generation-electrothermal atomic absorption spectrometry. Talanta 71:1834–1841
Morte ESB, Barbosa IS, Santos EC (2012) Axial view inductively coupled plasma optical emission spectrometry for monitoring tin concentration in canned tomato sauce samples. Food Chem 131:348–352
Pal BK, Ryan DE (1969) Fluorescence and metallic valency states: part III. Deter Tin Anal Chim Acta 48:227–231
Pasias IN, Papageorgiou V, Thomaidis NS, Proestos C (2012) Development and validation of an ETAAS method for the determination of tin in canned tomato paste samples. Food Anal Methods 5:835–840
Perring L, Basic-Dvorzak M (2002) Determination of total tin in canned food using inductively coupled plasma atomic emission spectroscopy. Anal Bioanal Chem 374:235–243
Qiong L, Guanghan L, Heng W, Xiaogang W (1999) Determination of trace tin in foods by single-sweep polarography. Food Chem 64:129–132
Ribeiro AS, Moretto AL, Arruda MAZ, Cadore S (2003) Analysis of powdered coffee and milk by ICP OES after sample treatment with tetramethylammonium hydroxide. Microchim Acta 141:149–155
Rose M, Baxter M, Brereton N, Baskaran C (2010) Dietary exposure to metals and other elements in the 2006 UK total diet study and some trends over the last 30 years. Food Add Contam A 27:1380–1404
Rubio S, Gómez-Hens A, Valcárcel M (1985) Fluorimetric determination of tin at the nanograms per millilitre level in canned beverages. Analyst 110:43–45
Taher MA, Puri BK (1999) Differential pulse polarographic determination of tin in alloys and environmental samples after preconcentration with the ion pair of 2-nitroso-1-naphthol-4-sulfonic acid and tetradecyldimethylbenzylammonium chloride onto microcrystalline naphthalene or by column method. Talanta 48:355–362
Tsogas GZ, Giokas DL, Vlessidis AG (2009) Graphite furnace and hydride generation atomic absorption spectrometric determination of cadmium, lead, and tin traces in natural surface waters: study of preconcentration technique performance. J Hazard Mater 163:988–994
Ulusoy S, Ulusoy Hİ, Akçay M, Gürkan R (2012) Inexpensive and versatile method for trace Sn(II) and Sn(IV) ions in food samples by CPE/FAAS. Food Chem 134:419–426
Weber G (1985) The importance of tin in the environment and its determination at trace levels. Fresenius J Anal Chem 321:217–224
Zhang Q, Minami H, Inoue S, Atsuya I (2001) Preconcentration by coprecipitation of arsenic and tin in natural waters with a Ni-pyrrolidine dithiocarbamate complex and their direct determination by solid-sampling atomic-absorption spectrometry. Fresenius J Anal Chem 370:860–864
Zhu X, Zhu X, Wang B (2006) Cloud point extraction for speciation analysis of inorganic tin in water samples by graphite furnace atomic absorption spectrometry. J Anal At Spectrom 21:69–73
Conflict of Interest
The authors have no conflict of interest. Our article does not contain any studies with human or animals subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amjadi, M., Manzoori, J.L. & Hamedpour, V. Optimized Ultrasound-Assisted Temperature-Controlled Ionic Liquid Microextraction Coupled with FAAS for Determination of Tin in Canned Foods. Food Anal. Methods 6, 1657–1664 (2013). https://doi.org/10.1007/s12161-013-9584-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-013-9584-x