Abstract
Experiments in series horizontal anaerobic reactors with fixed bed (HARFB) were conducted to evaluate the co-digestion of crude glycerol (CG) from biodiesel production and domestic sewage (DS) concerning the start-up strategy and best organic loading rate (OLR) to improve biogas production as well as to analyze the dynamic changes in the anaerobic microbial consortium during their operation. This approach can be used to increase the buffering capacity of anaerobic reactors as well as dilute toxic compounds. The reactor configuration applied at the present research is a novelty about CG and DS co-digestion and biogas production. The highest hydrogen generation was 10.3 mol H2 (m3 day−1) in reactor R1, and the highest methane yield was 312.0 and 283.9 L CH4 (m3 day−1) in R2 and R3, respectively. These values, obtained with mixed culture, agree with previous co-digestion research. The three-stage system showed high efficiency in removing crude glycerol and chemical oxygen demand (COD), with consumptions of 99.9%, both. The strategy of co-digestion is positive to avoid substrate inhibition. In addition, there was a change in the relative abundance of microorganisms among R1, R2, and R3 and a considerable decrease in the diversity index in the fermentation reactor (R1). The results have shown the potential of applying HARFB reactors for energy recovery and alternative waste disposal.
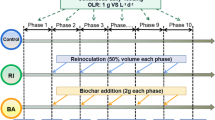
Graphical abstract




source and samples from R1, R2, and R3 at the end of Phase 1

Similar content being viewed by others
Data Availability
All experimental data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Kaur J, Sarma AK, Jha MK, Gera P (2020) Valorisation of crude glycerol to value-added products: perspectives of process technology, economics and environmental issues. Biotechnol Reports 27:e00487. https://doi.org/10.1016/j.btre.2020.e00487
Brazilian National Agency of Petroleum NG and B (2022) Oil, natural gas and biofuels statistical yearbook 2020. ANP/SDP. https://www.gov.br/anp/pt-br/centrais-de-conteudo/publicacoes/anuario-estatistico/oil-natural-gas-and-biofuels-statistical-yearbook-2020. Accessed 4 Feb 2022
Rivero M, Solera R, Perez M (2014) Anaerobic mesophilic co-digestion of sewage sludge with glycerol: Enhanced biohydrogen production. Int J Hydrog Energy 39:2481–2488. https://doi.org/10.1016/j.ijhydene.2013.12.006
Tangkathitipong P, Intanoo P, Butpan J (2017) Separate production of hydrogen and methane from biodiesel wastewater with added glycerin by two-stage anaerobic sequencing batch reactors (ASBR). Renew Energy 113:1077–1085. https://doi.org/10.1016/j.renene.2017.06.056
Rodrigues CV, Nespeca MG, Sakamoto IK et al (2019) Bioconversion of crude glycerol from waste cooking oils into hydrogen by sub-tropical mixed and pure cultures. Int J Hydrog Energy 4:144–154. https://doi.org/10.1016/j.ijhydene.2018.02.174
Sawasdee V, Haosagul S, Pisutpaisal N (2019) Co-digestion of waste glycerol and glucose to enhance biogas production. Int J Hydrog Energy 44:29575–29582. https://doi.org/10.1016/j.ijhydene.2019.03.144
Chilakamarry CR, Sakinah AMM, Zularisam AW et al (2021) Bioconversion of glycerol into biofuels—opportunities and challenges. BioEnergy Res. https://doi.org/10.1007/s12155-021-10353-6
Robles Á, Aguado D, Barat R et al (2020) New frontiers from removal to recycling of nitrogen and phosphorus from wastewater in the Circular Economy. Bioresour Technol 300:122673. https://doi.org/10.1016/j.biortech.2019.122673
Passos F, Bressani-Ribeiro T, Rezende S, Chernicharo CAL (2020) Potential applications of biogas produced in small-scale UASB-based sewage treatment plants in Brazil. Energies 13:3356. https://doi.org/10.3390/en13133356
Silva JA da, F.M. Braga A, Fermoso FG, et al (2021) Evaluation of the influence of trace metals on methane production from domestic sewage, using the Plackett-Burman experimental design. J Environ Manage 294:113002. https://doi.org/10.1016/j.jenvman.2021.113002
Rodrigues CV, Oliveira Santana K, Nespeca MG et al (2020) Energy valorization of crude glycerol and sanitary sewage in hydrogen generation by biological processes. Int J Hydrog Energy 45:11943–11953. https://doi.org/10.1016/j.ijhydene.2020.02.168
Sivagurunathan P, Kuppam C, Mudhoo A et al (2018) A comprehensive review on two-stage integrative schemes for the valorization of dark fermentative effluents. Crit Rev Biotechnol 38:868–882
da Silva Mazareli RC, Duda RM, Leite VD, de Oliveira RA (2016) Anaerobic co-digestion of vegetable waste and swine wastewater in high-rate horizontal reactors with fixed bed. Waste Manag 52:112–121. https://doi.org/10.1016/j.wasman.2016.03.021
Aydin S, Ince B, Ince O (2015) Application of real-time PCR to determination of combined effect of antibiotics on Bacteria, Methanogenic Archaea, Archaea in anaerobic sequencing batch reactors. Water Res 76:88–98. https://doi.org/10.1016/j.watres.2015.02.043
Pachiega R, Sakamoto IK, Varesche MB et al (2018) Obtaining and characterization of mesophilic bacterial consortia from tropical sludges applied on biohydrogen production. Waste Biomass Valorization. https://doi.org/10.1007/s12649-017-0185-6
Rossi DM, Berne Da Costa J, Aquino De Souza E et al (2011) Comparison of different pretreatment methods for hydrogen production using environmental microbial consortia on residual glycerol from biodiesel. Int J Hydrog Energy 36:4814–4819. https://doi.org/10.1016/j.ijhydene.2011.01.005
Rodrigues BCG, De Mello BS, Gonsales Da Costa Araujo ML, et al (2021) Soybean molasses as feedstock for sustainable generation of biomethane using high-rate anaerobic reactor. J Environ Chem Eng 9:105226. https://doi.org/10.1016/j.jece.2021.105226
Maintinguer S, Fernandes B, Duarte I et al (2008) Fermentative hydrogen production by microbial consortium. Int J Hydrog Energy 33:4309–4317. https://doi.org/10.1016/j.ijhydene.2008.06.053
Nation JL (1983) A new method using hexamethyldisilazane for preparation of soft insect tissues for scanning electron microscopy. Stain Technol 58:347–351. https://doi.org/10.3109/10520298309066811
Griffiths RI, Whiteley AS, O’Donnell AG, Bailey MJ (2000) Rapid method for coextraction of DNA and RNA from natural environments for analysis of ribosomal DNA- and rRNA-based microbial community composition. Appl Environ Microbiol 66:5488–5491. https://doi.org/10.1128/AEM.66.12.5488-5491.2000
Vasconcelos EAF, Santaella ST, Viana MB et al (2019) Composition and ecology of bacterial and archaeal communities in anaerobic reactor fed with residual glycerol. Anaerobe 59:145–153. https://doi.org/10.1016/j.anaerobe.2019.06.014
Jiraprasertwong A, Seneesrisakul K, Pornmai K, Chavadej S (2020) High methanogenic activity of a three-stage UASB in relation to the granular sludge formation. Sci Total Environ 724:138145. https://doi.org/10.1016/j.scitotenv.2020.138145
Sarma SJ, Brar SK, Sydney EB et al (2012) Microbial hydrogen production by bioconversion of crude glycerol: a review. Int J Hydrog Energy 37:6473–6490. https://doi.org/10.1016/j.ijhydene.2012.01.050
Selembo PA, Perez JM, Lloyd WA, Logan BE (2009) Enhanced hydrogen and 1,3-propanediol production from glycerol by fermentation using mixed cultures. Biotechnol Bioeng 104:1098–1106. https://doi.org/10.1002/bit.22487
Ngo TA, Kim MS, Sim SJ (2011) High-yield biohydrogen production from biodiesel manufacturing waste by Thermotoga neapolitana. Int J Hydrog Energy 36:5836–5842. https://doi.org/10.1016/j.ijhydene.2010.11.057
Veroneze ML, Schwantes D, Gonçalves AC et al (2019) Production of biogas and biofertilizer using anaerobic reactors with swine manure and glycerin doses. J Clean Prod 213:176–184. https://doi.org/10.1016/j.jclepro.2018.12.181
Veras STS, Rojas P, Florencio L et al (2019) Production of 1,3-propanediol from pure and crude glycerol using a UASB reactor with attached biomass in silicone support. Bioresour Technol 279:140–148. https://doi.org/10.1016/j.biortech.2019.01.125
Silva FMS, Mahler CF, Oliveira LB, Bassin JP (2018) Hydrogen and methane production in a two-stage anaerobic digestion system by co-digestion of food waste, sewage sludge and glycerol. Waste Manag 76:339–349. https://doi.org/10.1016/j.wasman.2018.02.039
Dinh NT (2020) A novel enhancement for the start-up of methane fermentation reactor by inoculating the acclimated sludge as a seeding material. IOP Conf Ser Mater Sci Eng 736:. https://doi.org/10.1088/1757-899X/736/4/042041
Filippidou S, Junier T, Wunderlin T et al (2015) Under-detection of endospore-forming Firmicutes in metagenomic data. Comput Struct Biotechnol J 13:299–306. https://doi.org/10.1016/j.csbj.2015.04.002
Ahlert S, Zimmermann R, Ebling J, König H (2016) Analysis of propionate-degrading consortia from agricultural biogas plants. Microbiologyopen 5:1027–1037. https://doi.org/10.1002/mbo3.386
Maintinguer SI, Fernandes BS, Duarte ICS et al (2011) Fermentative hydrogen production with xylose by Clostridium and Klebsiella species in anaerobic batch reactors. Int J Hydrog Energy 36:13508–13517. https://doi.org/10.1016/j.ijhydene.2011.07.095
Wu Y, Wang C, Liu X et al (2016) A new method of two-phase anaerobic digestion for fruit and vegetable waste treatment. Bioresour Technol 211:16–23. https://doi.org/10.1016/j.biortech.2016.03.050
Zhao Y, Wu J, Yuan X et al (2017) The effect of mixing intensity on the performance and microbial dynamics of a single vertical reactor integrating acidogenic and methanogenic phases in lignocellulosic biomass digestion. Bioresour Technol 238:542–551. https://doi.org/10.1016/j.biortech.2017.04.080
Castelló E, García y Santos C, Iglesias T et al (2009) Feasibility of biohydrogen production from cheese whey using a UASB reactor: links between microbial community and reactor performance. Int J Hydrog Energy 34:5674–5682. https://doi.org/10.1016/j.ijhydene.2009.05.060
Song J, An D, Ren N et al (2011) Effects of pH and ORP on microbial ecology and kinetics for hydrogen production in continuously dark fermentation. Bioresour Technol 102:10875–10880. https://doi.org/10.1016/j.biortech.2011.09.024
Li C, Zhang T, Fang HHP (2006) Fermentative hydrogen production in packed-bed and packaging-free upflow reactors. Water Sci Technol 54:95–103. https://doi.org/10.2166/wst.2006.712
Luo Y, Zhang H, Salerno M et al (2008) Organic loading rates affect composition of soil-derived bacterial communities during continuous, fermentative biohydrogen production. Int J Hydrog Energy 33:6566–6576. https://doi.org/10.1016/j.ijhydene.2008.08.047
Bonin AS, Boone DR (2006) The order methanobacteriales characteristics of methanobacteriales. Prokaryotes 3:231–243. https://doi.org/10.1007/0-387-30743-5_11
da Silva AN, Macêdo WV, Sakamoto IK et al (2019) Biohydrogen production from dairy industry wastewater in an anaerobic fluidized-bed reactor. Biomass Bioenerg 120:257–264. https://doi.org/10.1016/j.biombioe.2018.11.025
Zhao C, Karakashev D, Lu W et al (2010) Xylose fermentation to biofuels (hydrogen and ethanol) by extreme thermophilic (70 °C) mixed culture. Int J Hydrog Energy 35:3415–3422. https://doi.org/10.1016/j.ijhydene.2010.01.082
Maintinguer SI, Sakamoto IK, Adorno MAT, Varesche MBA (2015) Bacterial diversity from environmental sample applied to bio-hydrogen production. Int J Hydrog Energy 40:3180–3190. https://doi.org/10.1016/j.ijhydene.2014.12.118
Abreu AA, Alves JI, Pereira MA et al (2011) Strategies to suppress hydrogen-consuming microorganisms affect macro and micro scale structure and microbiology of granular sludge. Biotechnol Bioeng 108:1766–1775. https://doi.org/10.1002/bit.23145
Marzorati M, Wittebolle L, Boon N et al (2008) How to get more out of molecular fingerprints: Practical tools for microbial ecology. Environ Microbiol 10:1571–1581. https://doi.org/10.1111/j.1462-2920.2008.01572.x
Lebrero R, Rodríguez E, Pérez R et al (2013) Abatement of odorant compounds in one- and two-phase biotrickling filters under steady and transient conditions. Appl Microbiol Biotechnol 97:4627–4638. https://doi.org/10.1007/s00253-012-4247-1
Traversi D, Villa S, Lorenzi E et al (2012) Application of a real-time qPCR method to measure the methanogen concentration during anaerobic digestion as an indicator of biogas production capacity. J Environ Manage 111:173–177. https://doi.org/10.1016/j.jenvman.2012.07.021
Maintinguer SI, Sakamoto IK, Adorno MAT, Varesche MBA (2016) Diversity of anaerobic bacteria in sediments from a subtropical reservoir. Lakes Reserv Res Manag 21:351–361. https://doi.org/10.1111/lre.12156
Baloch MI, Akunna JC, Kierans M, Collier PJ (2008) Structural analysis of anaerobic granules in a phase separated reactor by electron microscopy. Bioresour Technol 99:922–929. https://doi.org/10.1016/j.biortech.2007.03.016
Haosagul S, Vikromvarasiri N, Sawasdee V, Pisutpaisal N (2019) Impact of acetic acid in methane production from glycerol/acetic acid co-fermentation. Int J Hydrog Energy 44:29568–29574. https://doi.org/10.1016/j.ijhydene.2019.03.204
Sittijunda S, Reungsang A (2017) Fermentation of hydrogen, 1,3-propanediol and ethanol from glycerol as affected by organic loading rate using up-flow anaerobic sludge blanket (UASB) reactor. Int J Hydrog Energy 42:27558–27569. https://doi.org/10.1016/j.ijhydene.2017.05.149
Acknowledgements
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ) under Grant 407298/2018-5 and 457144/2014-9; and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) under Grant 2017/11767-1 and 2017/25329-6. The authors are also grateful to the “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil” (CAPES) – Finance Code 001.
Funding
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ) under Grant 407298/2018–5 and 457144/2014–9; and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) under Grant 2017/11767–1 and 2017/25329–6. The authors are also grateful to the “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil” (CAPES) – Finance Code 001.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Luan Vieira Adames, Ana Paula Jacobus, and Isabel Sakamoto performed material preparation, data collection, and analysis. Sandra Imaculada Maintinguer and Lorena Oliveira Pires made funding acquisition, advising, and experimental outline. Luan Vieira Adames wrote the first draft of the manuscript. Discussion, English, and text revised by Carolina Zampol Lazaro. All authors commented on previous versions of the manuscript read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Adames, L.V., Jacobus, A.P., Sakamoto, I.K. et al. Bioenergy Recovery from Anaerobic Co-Digestion of Crude Glycerol and Domestic Sewage In-Series Reactor: Microbial Characterization and System Performance. Bioenerg. Res. 15, 2145–2158 (2022). https://doi.org/10.1007/s12155-022-10417-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-022-10417-1