Abstract
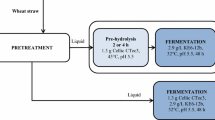
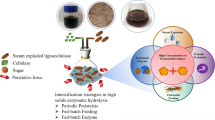
Converting the chemical and biologically resistant structure of cellulosic biomass into useful products is a technological and economic challenge. The use of sustainable and efficient conversion technology sustainably has been gaining prominence. This work aimed to evaluate the integration of subcritical water hydrolysis (SWH) and enzymatic hydrolysis of sugarcane straw to obtain fermentable products and second-generation ethanol. SWH was conducted under semi-continuous conditions at 200 °C, 5 mL/min, and a 19-min retention time. Enzymatic hydrolysis using an enzyme blend produced simple sugars that were evaluated for fermentation using two strategies: (i) Saccharomyces cerevisiae SA-1; and (ii) co-culture of S. cerevisiae SA-1 and Scheffersomyces stipitis NRRLY-7124. Integrating subcritical water and enzymatic hydrolysis resulted in greater fermentable sugar yield (2.40 ± 0.01 g/L of xylose and 8.9 ± 0.6 g/L of glucose) than subcritical pre-treatment on its own. The co-culture fermentation strategy was more efficient than fermentation by S. cerevisiae on its own, resulting in 2.8 ± 0.3 g/L of ethanol. Subcritical water hydrolysis pre-treatment disrupted the lignocellulosic matrix, leading to increased enzymatic accessibility of cellulose, with minimal formation of degradation compounds, and with minimal waste formation. Future work can optimize the hydrolysis process to increase the yield of simple sugars, increase fermentation efficiency by controlling oxygen concentration and removing acetic acid, and consequently increase the yields of second-generation ethanol.
Graphical abstract





Similar content being viewed by others
References
Rastogi M, Shrivastava S (2017) Recent advances in second generation bioethanol production: An insight to pretreatment, saccharification and fermentation processes. Renew Sust Energ Rev 80:330–340. https://doi.org/10.1016/j.rser.2017.05.225
Lachos-Perez D, Tompsett G, Guerra P, Timko M, Rostagno M, Martínez J, Forster-Carneiro T (2017) Sugars and char formation on subcritical water hydrolysis of sugarcane straw. Bioresour Technol 243:1069–1077. https://doi.org/10.1016/j.biortech.2017.07.080
Zabed H, Sahu J, Boyce AN, Faruq G (2016) Fuel ethanol production from lignocellulosic biomass: an overview on feedstocks and technological approaches. Renew Sust Energ Rev 66:751–774. https://doi.org/10.1016/j.rser.2016.08.038
Basu P (2013) Chapter 3 - Biomass characteristics. In: Basu P (ed) Biomass gasification, pyrolysis and torrefaction, 2nd edn. Academic Press, Boston, pp 47–86. https://doi.org/10.1016/B978-0-12-396488-5.00003-4
Prado JM, Follegatti-Romero LA, Forster-Carneiro T, Rostagno MA, Maugeri Filho F, Meireles MAA (2014) Hydrolysis of sugarcane bagasse in subcritical water. J Supercrit Fluids 86:15–22. https://doi.org/10.1016/j.supflu.2013.11.018
Candido RG, Mori NR, Goncalves AR (2019) Sugarcane straw as feedstock for 2G ethanol: evaluation of pretreatments and enzymatic hydrolysis. Ind Crop Prod 142:111845. https://doi.org/10.1016/j.indcrop.2019.111845
Moro MK, Teixeira RSS, da Silva ASA, Fujimoto MD, Melo PA, Secchi AR, da Silva Bon EP (2017) Continuous pretreatment of sugarcane biomass using a twin-screw extruder. Ind Crop Prod 97:509–517. https://doi.org/10.1016/j.indcrop.2016.12.051
Ávila PF, Forte MB, Goldbeck R (2018) Evaluation of the chemical composition of a mixture of sugarcane bagasse and straw after different pretreatments and their effects on commercial enzyme combinations for the production of fermentable sugars. Biomass Bioenergy 116:180–188. https://doi.org/10.1016/j.biombioe.2018.06.015
Silveira MHL, Chandel AK, Vanelli BA, Sacilotto KS, Cardoso EB (2018) Production of hemicellulosic sugars from sugarcane bagasse via steam explosion employing industrially feasible conditions: Pilot scale study. Bioresour Technol Rep 3:138–146. https://doi.org/10.1016/j.biteb.2018.07.011
de Carvalho Silvello MA, Martínez J, Goldbeck R (2019) Increase of reducing sugars release by enzymatic hydrolysis of sugarcane bagasse intensified by ultrasonic treatment. Biomass Bioenergy 122:481–489. https://doi.org/10.1016/j.biombioe.2019.01.032
Lyu H, Zhou J, Geng Z, Lyu C, Li Y (2018) Two-stage processing of liquid hot water pretreatment for recovering C5 and C6 sugars from cassava straw. Process Biochem 75:202–211. https://doi.org/10.1016/j.procbio.2018.10.003
Yang XW, Cui CX, Zheng AQ, Zhao ZL, Wang CY, Xia SP, Huang Z, Wei GQ, Li HB (2020) Ultrasonic and microwave assisted organosolv pretreatment of pine wood for producing pyrolytic sugars and phenols. Ind Crop Prod 157:10. https://doi.org/10.1016/j.indcrop.2020.112921
Mello L, Mali S (2020) A combination of chemical and physical pretreatments in the saccharification of malt bagasse: the effects of ultrasonication in diluted acid medium. Biomass Convers Bior:12. https://doi.org/10.1007/s13399-020-01106-0
Hosgun EZ, Ay SB, Bozan B (2020) Effect of sequential pretreatment combinations on the composition and enzymatic hydrolysis of hazelnut shells. Prep Biochem Biotechnol 10. https://doi.org/10.1080/10826068.2020.1836657
Dai NH, Huynh KTT, Nguyen TAD, Do VVT, Van Tran M (2020) Hydrothermal and steam explosion pretreatment of Bambusa stenostachya bamboo. Waste Biomass Valoriz:1–10. https://doi.org/10.1007/s12649-020-01299-5
Mayanga-Torres P, Lachos-Perez D, Rezende C, Prado J, Ma Z, Tompsett G, Timko M, Forster-Carneiro T (2017) Valorization of coffee industry residues by subcritical water hydrolysis: recovery of sugars and phenolic compounds. J Supercrit Fluids 120:75–85. https://doi.org/10.1016/j.supflu.2016.10.015
Torres-Mayanga PC, Azambuja SPH, Tyufekchiev M, Tompsett GA, Timko MT, Goldbeck R, Rostagno MA, Forster-Carneiro T (2019) Subcritical water hydrolysis of brewer’s spent grains: selective production of hemicellulosic sugars (C-5 sugars). J Supercrit Fluids 145:19–30. https://doi.org/10.1016/j.supflu.2018.11.019
Lin R, Cheng J, Ding L, Song W, Qi F, Zhou J, Cen K (2015) Subcritical water hydrolysis of rice straw for reducing sugar production with focus on degradation by-products and kinetic analysis. Bioresour Technol 186:8–14. https://doi.org/10.1016/j.biortech.2015.03.047
Vedovatto F, Ugalde G, Bonatto C, Bazoti SF, Treichel H, Mazutti MA, Zabot GL, Tres MV (2021) Subcritical water hydrolysis of soybean residues for obtaining fermentable sugars. J Supercrit Fluids 167:12. https://doi.org/10.1016/j.supflu.2020.105043
Abaide ER, Mortari SR, Ugalde G, Valério A, Amorim SM, Di Luccio M, Moreira RFPM, Kuhn RC, Priamo WL, Tres MV, Zabot GL, Mazutti MA (2019) Subcritical water hydrolysis of rice straw in a semi-continuous mode. J Clean Prod 209:386–397. https://doi.org/10.1016/j.jclepro.2018.10.259
Abaide ER, Ugalde G, Di Luccio M, Moreira RFPM, Tres MV, Zabot GL, Mazutti MA (2019) Obtaining fermentable sugars and bioproducts from rice husks by subcritical water hydrolysis in a semi-continuous mode. Bioresour Technol 272:510–520. https://doi.org/10.1016/j.biortech.2018.10.075
Cocero MJ, Cabeza A, Abad N, Adamovic T, Vaquerizo L, Martinez CM, Pazo-Cepeda MV (2018) Understanding biomass fractionation in subcritical & supercritical water. J Supercrit Fluids 133:550–565. https://doi.org/10.1016/j.supflu.2017.08.012
Ruiz HA, Conrad M, Sun S-N, Sanchez A, Rocha GJM, Romaní A, Castro E, Torres A, Rodríguez-Jasso RM, Andrade LP, Smirnova I, Sun R-C, Meyer AS (2020) Engineering aspects of hydrothermal pretreatment: From batch to continuous operation, scale-up and pilot reactor under biorefinery concept. Bioresour Technol 299:122685. https://doi.org/10.1016/j.biortech.2019.122685
Prado JM, Lachos-Perez D, Forster-Carneiro T, Rostagno MA (2016) Sub-and supercritical water hydrolysis of agricultural and food industry residues for the production of fermentable sugars: a review. Food Bioprod Process 98:95–123. https://doi.org/10.1016/j.fbp.2015.11.004
Ciftci D, Saldaña MDA (2015) Hydrolysis of sweet blue lupin hull using subcritical water technology. Bioresour Technol 194:75–82. https://doi.org/10.1016/j.biortech.2015.06.146
Pedras BM, Nascimento M, Sá-Nogueira I, Simões P, Paiva A, Barreiros S (2019) Semi-continuous extraction/hydrolysis of spent coffee grounds with subcritical water. J Ind Eng Chem 72:453–456. https://doi.org/10.1016/j.jiec.2019.01.001
Sasaki M, Fang Z, Fukushima Y, Adschiri T, Arai K (2000) Dissolution and hydrolysis of cellulose in subcritical and supercritical water. Ind Eng Chem Res 39(8):2883–2890. https://doi.org/10.1021/ie990690j
Ma Z, Guerra P, Tyufekchiev M, Zaker A, Tompsett GA, Mayanga PT, Forster-Carneiro T, Wang P, Timko MT (2017) Formation of an external char layer during subcritical water hydrolysis of biomass. Sustain Energy Fuels 1(9):1950–1959. https://doi.org/10.1039/C7SE00260B
Oliveira TCG, Hanlon KE, Interlandi MA, Torres-Mayanga PC, Silvello MAC, Lachos-Perez D, Timko MT, Rostagno MA, Goldbeck R, Forster-Carneiro T (2020) Subcritical water hydrolysis pretreatment of sugarcane bagasse to produce second generation ethanol. J Supercrit Fluids 164:104916. https://doi.org/10.1016/j.supflu.2020.104916
Pengilly C, García-Aparicio MP, Diedericks D, Brienzo M, Görgens JF (2015) Enzymatic hydrolysis of steam-pretreated sweet sorghum bagasse by combinations of cellulase and endo-xylanase. Fuel 154:352–360. https://doi.org/10.1016/j.fuel.2015.03.072
Gomes MG, Gurgel LVA, Baffi MA, Pasquini D (2020) Pretreatment of sugarcane bagasse using citric acid and its use in enzymatic hydrolysis. Renew Energy 157:332–341. https://doi.org/10.1016/j.renene.2020.05.002
Lin Y, Tanaka S (2006) Ethanol fermentation from biomass resources: current state and prospects. Appl Microbiol Biotechnol 69(6):627–642. https://doi.org/10.1007/s00253-005-0229-x
Aditiya H, Mahlia T, Chong W, Nur H, Sebayang A (2016) Second generation bioethanol production: a critical review. Renew Sust Energ Rev 66:631–653. https://doi.org/10.1016/j.rser.2016.07.015
Teixeira VS, Azambuja SPH, Carvalho PH, Costa FAA, Kitaka PR, Steckelberg C, Andrietta SR, Andrietta MGS, Goldbeck R (2019) Robustness and ethanol production of industrial strains of Saccharomyces cerevisiae using different sugarcane bagasse hydrolysates. J Appl Biotechnol 7(1). https://doi.org/10.5296/jab.v7i1.14599
Farias D, de Andrade RR, Maugeri-Filho F (2014) Kinetic modeling of ethanol production by Scheffersomyces stipitis from xylose. Appl Biochem Biotechnol 172(1):361–379. https://doi.org/10.1007/s12010-013-0546-y
José C, Lino AG, Colodette JL, Lima CF, Gutiérrez A, Martínez ÁT, Lu F, Ralph J, Rencoret J (2015) Differences in the chemical structure of the lignins from sugarcane bagasse and straw. Biomass Bioenergy 81:322–338. https://doi.org/10.1016/j.biombioe.2015.07.006
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D (2005) Determination of total solids in biomass. NREL Biomass Analysis Technol Team Lab Analy Proced 1
Sluiter A, Ruiz R, Scarlata C, Sluiter J, Templeton D (2005) Determination of extractives in biomass. Lab Analy Proced (LAP) 1617
Hames B, Scarlata C, Sluiter A (2008) Determination of protein content in biomass. Nat Renew Energ Lab:1–5
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D (2008) Determination of ash in biomass. National Renew Energ Lab (NREL/TP-510-42622)
Hyman D, Sluiter A, Crocker D, Johnson D, Sluiter J, Black S, Scarlata C (2008) Determination of acid soluble lignin concentration curve by UV-Vis spectroscopy. Natl Renew Energ Lab (NREL) Analytical Proced, pp 1-13
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2008) Determination of structural carbohydrates and lignin in biomass. Lab Analy Proced 1617:1–16
Sluiter JB, Chum H, Gomes AC, Tavares R, Azevedo V, Pimenta MT, Rabelo SC, Marabezi K, Curvelo AA, Alves AR (2016) Evaluation of Brazilian sugarcane bagasse characterization: an interlaboratory comparison study. J AOAC Internat 99(3):579–585. https://doi.org/10.5740/jaoacint.15-0063
Wagner W, Pruß A (2002) The IAPWS formulation 1995 for the thermodynamic properties of ordinary water substance for general and scientific use. J Phys Chem Ref Data 31(2):387–535. https://doi.org/10.1063/1.1461829
de Carvalho Silvello MA, Martínez J, Goldbeck R (2020) Alternative technology for intensification of fermentable sugars released from enzymatic hydrolysis of sugarcane bagasse. Biomass Conversi Bior. https://doi.org/10.1007/s13399-020-00752-8
de Carvalho Silvello MA, Martínez J, Goldbeck R (2020) Application of supercritical CO 2 treatment enhances enzymatic hydrolysis of sugarcane bagasse. BioEnergy Res 13(3):786–796. https://doi.org/10.1007/s12155-020-10130-x
Costa SM, Mazzola PG, Silva JC, Pahl R, Pessoa A Jr, Costa SA (2013) Use of sugar cane straw as a source of cellulose for textile fiber production. Ind Crop Prod 42:189–194. https://doi.org/10.1016/j.indcrop.2012.05.028
da Silva ASA, Inoue H, Endo T, Yano S, Bon EP (2010) Milling pretreatment of sugarcane bagasse and straw for enzymatic hydrolysis and ethanol fermentation. Bioresour Technol 101(19):7402–7409. https://doi.org/10.1016/j.biortech.2010.05.008
Szczerbowski D, Pitarelo AP, Zandoná Filho A, Ramos LP (2014) Sugarcane biomass for biorefineries: comparative composition of carbohydrate and non-carbohydrate components of bagasse and straw. Carbohydr Polym 114:95–101. https://doi.org/10.1016/j.carbpol.2014.07.052
Lachos-Perez D, Martinez-Jimenez F, Rezende CA, Tompsett G, Timko M, Forster-Carneiro T (2016) Subcritical water hydrolysis of sugarcane bagasse: an approach on solid residues characterization. J Supercrit Fluids 108:69–78. https://doi.org/10.1016/j.supflu.2015.10.019
Archambault-Léger V, Losordo Z, Lynd LR (2015) Energy, sugar dilution, and economic analysis of hot water flow-through pre-treatment for producing biofuel from sugarcane residues. Biofuels Bioprod Biorefin 9(1):95–108. https://doi.org/10.1002/bbb.1524
Mussatto SI, Roberto IC (2004) Alternatives for detoxification of diluted-acid lignocellulosic hydrolyzates for use in fermentative processes: a review. Bioresour Technol 93(1):1–10. https://doi.org/10.1016/j.biortech.2003.10.005
Wikandari R, Sanjaya AP, Millati R, Karimi K, Taherzadeh MJ (2019) Fermentation inhibitors in ethanol and biogas processes and strategies to counteract their effects. Biofuels: Alternative Feedstocks and Conversion Processes for the Production of Liquid and Gaseous Biofuels. Elsevier, pp 461-499. https://doi.org/10.1002/maco.202070074
Ko JK, Um Y, Lee S-M (2016) Effect of manganese ions on ethanol fermentation by xylose isomerase expressing Saccharomyces cerevisiae under acetic acid stress. Bioresour Technol 222:422–430. https://doi.org/10.1016/j.biortech.2016.09.130
Weingarten R, Conner WC, Huber GW (2012) Production of levulinic acid from cellulose by hydrothermal decomposition combined with aqueous phase dehydration with a solid acid catalyst. Energy Environ Sci 5(6):7559–7574. https://doi.org/10.1039/C2EE21593D
Wang X, Gao L, Liu L, Wang T, Yin H, Lü X (2018) A novel closed-circuit circulation system about integrated ethanol-methane fermentation process based on the subcritical water pretreatment of corn stover. J Clean Prod 180:472–481. https://doi.org/10.1016/j.jclepro.2018.01.196
Helle S, Cameron D, Lam J, White B, Duff S (2003) Effect of inhibitory compounds found in biomass hydrolysates on growth and xylose fermentation by a genetically engineered strain of S. cerevisiae. Enzym Microb Technol 33(6):786–792. https://doi.org/10.1016/S0141-0229(03)00214-X
Jiang W, Chang S, Li H, Oleskowicz-Popiel P, Xu J (2015) Liquid hot water pretreatment on different parts of cotton stalk to facilitate ethanol production. Bioresour Technol 176:175–180. https://doi.org/10.1016/j.biortech.2014.11.023
Liu ZL, Slininger PJ, Gorsich SW (2005) Enhanced biotransformation of furfural and hydroxymethylfurfural by newly developed ethanologenic yeast strains. Appl Biochem Biotechnol 121(1-3):451–460. https://doi.org/10.1385/ABAB:121:1-3:0451
Sunitha K, Lee J-K, Oh T-K (1999) Optimization of medium components for phytase production by E. coli using response surface methodology. Bioprocess Eng 21(6):477–481. https://doi.org/10.1007/PL00009086
Huang Y, Qin X, Luo X-M, Nong Q, Yang Q, Zhang Z, Gao Y, Lv F, Chen Y, Yu Z (2015) Efficient enzymatic hydrolysis and simultaneous saccharification and fermentation of sugarcane bagasse pulp for ethanol production by cellulase from Penicillium oxalicum EU2106 and thermotolerant Saccharomyces cerevisiae ZM1-5. Biomass Bioenergy 77:53–63. https://doi.org/10.1016/j.biombioe.2015.03.020
Maciel-Silva FW, Mussatto SI, Forster-Carneiro T (2019) Integration of subcritical water pretreatment and anaerobic digestion technologies for valorization of açai processing industries residues. J Clean Prod 228:1131–1142. https://doi.org/10.1016/j.jclepro.2019.04.362
Tao L, Schell D, Davis R, Tan E, Elander R, Bratis A (2014) NREL 2012 achievement of ethanol cost targets: biochemical ethanol fermentation via dilute-acid pretreatment and enzymatic hydrolysis of corn stover. National Renew Energ Lab (NREL), Golden, CO (United States). https://doi.org/10.2172/1129271
Funding
The authors received financial support from the Higher Level Personnel Improvement Coordination (CAPES) – Brazil- Finance code 001. The authors also received financial support from the São Paulo Research Foundation – FAPESP (2013/04304-4; 2016/50612-8; 2018/05999-0; 2018/14938-4; 2018/14582-5; 2019/08542-3). T. Forster-Carneiro is a recipient of the CNPq productivity grant (302473/2019-0). “Research supported by Pilot Plant for Process Development from CTBE – Brazilian Bioethanol Science and Technology Laboratory, CNPEM/MCTIC.” WPI’s contributions were supported by the US National Science Foundation (ENG #1554283).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Subcritical water and enzymatic hydrolysis combined provided the high sugar yield.
• The hydrolyzate for fermentation has 2.40 ± 0.01 g/L of xylose and 8.93 ± 0.60 g/L of glucose.
• The hydrolyzate glucose was consumed, and about 50% of the original xylose was consumed.
• The process has promise for the production of 2G-ethanol from sugarcane straw.
Rights and permissions
About this article
Cite this article
Oliveira, T.C.G., Interlandi, M.A., Hanlon, K.E. et al. Integration of Subcritical Water and Enzymatic Hydrolysis to Obtain Fermentable Sugars and Second-Generation Ethanol from Sugarcane Straw. Bioenerg. Res. 15, 1071–1082 (2022). https://doi.org/10.1007/s12155-021-10274-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-021-10274-4