Abstract
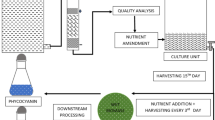
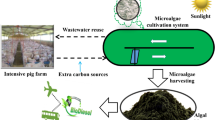
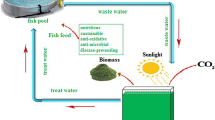
The rapid development of large-scale aquaculture leads to wastewater accumulation, increasing environmental problems. Microalga cultivation is a potential biotechnological alternative to treat aquaculture wastewater. While this microorganism consumes the wastewater nutrients, high added value biomass is produced. The role of Spirulina in aquaculture wastewater treatment is not fully elucidated in the literature. Thus, this study aimed to reuse and treat aquaculture wastewater by Spirulina sp. LEB 18 cultures. The microalga growth parameters, the biochemical composition of the biomass produced, and the Spirulina efficiency to nutrient removal from the aquaculture wastewater were evaluated. The assays were performed in closed photobioreactors (1 L) using 100% aquaculture wastewater (T-0) supplemented with 25 (T-25), 50 (T-50), and 75% (T-75) of the Zarrouk synthetic culture medium. The maximum biomass concentrations showed no statistical difference between the assays T-50 (1.02 g L−1), T-25 (1.10 g L−1), and control (1.05 g L−1). The biomass from the T-25 assay showed the highest concentrations of protein (65.73%), phycocyanin (16.60 mg/mL), polyunsaturated fatty acid (38.20%), and γ-linolênico (23.29%). Besides that, the Spirulina sp. LEB 18 highest removal rate of sulfate (94.01%), phosphate (93.84%), bromine (96.77%), and COD (90.00%) was obtained from the T-25 assay. The biomass from T-25 and T-50 assays showed ideal properties for biodiesel application. The Spirulina sp. LEB 18 cultures using 100% aquaculture wastewater supplemented with 25% of Zarrouk culture medium was the best option for the aquaculture wastewater treatment, producing added value biomass and reducing production cost.



Similar content being viewed by others
References
FAO (2018) Food and Agriculture Organization of the United Nations. The State of World Fisheries and Aquaculture. Rome:223
Wuang SC, Khin MC, Chua PQD, Luo YD (2016) Use of Spirulina biomass produced from treatment of aquaculture wastewater as agricultural fertilizers. Microalgae Res 15:59–64. https://doi.org/10.1016/j.algal.2016.02.009
Ferreira JG, Falconer L, Kittiwanich J, Ross L, Saurel C, Wellman K, Zhu CB, Suvanachai P (2015) Analysis of production and environmental effects of Nile tilapia and white shrimp culture in Thailand. Aquac Res 447:23–36. https://doi.org/10.1016/j.aquaculture.2014.08.042
Fitwi BS, Wuertz S, Schroeder JP, Schulz C (2012) Sustainability assessment tools to support aquaculture development. Aquac Res 32:183–192. https://doi.org/10.1016/j.jclepro.2012.03.037
Martins AP, Zambotti-Villela L, Yokoya NS, Colepicolo P (2018) Biotechnological potential of benthic marine algae collected along the Brazilian coast. Microalgae Res 33:316–327. https://doi.org/10.1016/j.algal.2018.05.008
Huang Y, Chen Y, Xie I, Liu H, Yin X, Wu C (2016) Bio-oil production from hydrothermal liquefaction of high-protein high-ash microalgae including wild Cyanobacteria sp. and cultivated Bacillariophyta sp. Fuel 183:9–19. https://doi.org/10.1016/j.fuel.2016.06.013
Markou G, Chatzipavlidis I, Georgakakis D (2012) Cultivation of Arthrospira (Spirulina platensis) in olive-oil mill wastewater treated with sodium hypochlorite. Bioresour Technol 112:234–241. https://doi.org/10.1016/j.biortech.2012.02.098
Alva MS, Pabella VML, Ledesma MTO, Gómez MJC (2018) Carbon, nitrogen, and phosphorus removal, and lipid production by three saline microalgae grown in synthetic wastewater irradiated with different photon fluxes. Microalgae Res 34:97–103. https://doi.org/10.1016/j.algal.2018.07.006
Kuo C, Chen TY, Lin TH, Kao CY, Lai JT, Chang JS, Lin CS (2015) Cultivation of Chlorella sp., GD using piggery wastewater for biomass and lipid production. Bioresour Technol 194:326–333. https://doi.org/10.1016/j.biortech.2015.07.026
Salama E, Jeon BH, Chang SW, Lee SH, Roh HS, Yang S, Kurade MB, El-Dalatony MM, Kim KH, Kim S (2017) Interactive effect of indole-3-acetic acid and diethyl aminoethyl hexanoate on the growth and fatty acid content of some microalgae for biodiesel production. J Clean 168:1017–1024. https://doi.org/10.1016/j.jclepro.2017.09.057
Xin L, Hong-ying H, Ke G, Ying-xue S (2010) Effects of different nitrogen and phosphorus concentrations on the growth, nutrient uptake, and lipid accumulation of a freshwater microalga Scenedesmus sp. Bioresour Technol 101:5494–5500. https://doi.org/10.1016/j.biortech.2010.02.016
Zhang L, Pei H, Yang Z, Wang X, Chen S, Li Y, Xie Z (2019) Microalgae nourished by mariculture wastewater aids aquaculture self-reliance with desirable biochemical composition. Bioresour Technol 278:205–213. https://doi.org/10.1016/j.biortech.2019.01.066
Andrade BB, Cardoso LG, Assis DJ, Costa JAV, Druzian JI, Lima STC (2019) Production and characterization of Spirulina sp. LEB 18 cultured in reused Zarrouk’s medium in a raceway-type bioreactor. Bioresour Technol 284:340–348. https://doi.org/10.1016/j.biortech.2019.03.144
Duarte JH, Cardoso LG, Souza CO, Nunes IL, Druzian JI, Morais MG, Costa JAV (2019) Brackish groundwater from Brazilian backlands in Spirulina cultures: potential of carbohydrate and polyunsaturated fatty acid production. Appl Biochem Biotechnol 190:907–917. https://doi.org/10.1007/s12010-019-03126-7
Mata SN, Cardoso LG, Andrade BB, Duarte JH, Costa JAV, Druzian JI (2020) Spirulina sp. LEB 18 cultivation in a raceway-type bioreactor using wastewater from desalination process: production of carbohydrate-rich biomass. Bioresour Technol 311:–123495. https://doi.org/10.1016/j.biortech.2020.123495
Costa JAV, Colla LM, Filho PD, Kabke K, Weber A (2004) Modelling of Spirulina platensis growth in fresh water using response surface methodology. J Microbiol Biotechnol 18:603–607. https://doi.org/10.1023/A:1016822717583
Daneshvar E, Antikainen L, Koutra E, Kornaros M, Bhatnagar A (2018) Investigation on the feasibility of Chlorella vulgaris cultivation in a mixture of pulp and aquaculture effluents: treatment of wastewater and lipid extraction. Bioresour Technol 255:104–110. https://doi.org/10.1016/j.biortech.2018.01.101
American Public Health Association (2005) Standard methods for the examination of water and wastewater. APHA, Washington
Soares SAR, Costa CR, Araujo RGO, Zucchi MR, Celino JJ, Teixeira LSG (2015) Determination of polycyclic aromatic hydrocarbons in groundwater samples by gas chromatography-mass spectrometry after pre-concentration using cloud-point extraction with surfactant Derivatization. J Braz Chem Soc 26:955–962. https://doi.org/10.5935/0103-5053.20150057
Ramsundar P, Abhishek G, Singh P, Pillay K, Bux F (2017) Evaluation of water activated sludge as a potential nutrient source for cultivation of Chlorella sorokiniana. Microalgae Res 28:108–117. https://doi.org/10.1016/j.algal.2017.10.006
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. Int J Biol Chem 193:265–275
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. https://doi.org/10.1021/ac60111a017
Nascimento IA, Marques SSI, Cabanelas ITD, Carvalho GC, Nascimento MA, Souza CO, Druzian JI, Hussain J, Liao W (2014) Microalgae versus land crops as feedstock for biodiesel: productivity, quality and standard compliance. Bioenergy Res 7:1002–1013. https://doi.org/10.1007/s12155-014-9440-x
Zhang L, Cheng J, Pei H, Pan J, Jiang L, Hou Q, Han F (2018) Cultivation of microalgae using anaerobically digested effluent from kitchen waste as a nutrient source for biodiesel production. Renew Energy 115:276–287. https://doi.org/10.1016/j.renene.2017.08.034
Milhazes-Cunha H, Otero A (2017) Valorisation of aquaculture effluents with microalgae: the integrated multi-trophic aquaculture concept. Algal Res 24:416–424. https://doi.org/10.1016/j.algal.2016.12.011
Malibari R, Sayegh F, Elazzazy AM, Baeshen MN, Dourou M, Aggelis G (2018) Reuse of shrimp farm wastewater as growth medium for marine microalgae isolated from Red Sea e Jeddah. J Clean 198:160–169. https://doi.org/10.1016/j.jclepro.2018.07.037
Ansari FA, Singh P, Guldhe A, Bux F (2017) Microalgal cultivation using aquaculture wastewater: integrated biomass generation and nutrient remediation. Algal Res 21:169–177. https://doi.org/10.1016/j.algal.2016.11.015
Mohammadi M, Mowla D, Esmaeilzadeh F, Ghasemi Y (2019) Enhancement of sulfate removal from the power plant wastewater using cultivation of indigenous microalgae: stage-wise operation. J Environ Chem Eng 7:102870. https://doi.org/10.1016/j.jece.2018.102870
Krishnamoorthya S, Manickam M, Muthukaruppanb V (2019) Evaluation of distillery wastewater treatability in a customized photobioreactor using blue-green microalgae – laboratory and outdoor study. J Environ Manag 234:412–423. https://doi.org/10.1016/j.jenvman.2019.01.014
Lu W, Alam MA, Luo W, Asmatulu E (2019) Integrating Spirulina platensis cultivation and aerobic composting exhaust for carbon mitigation and biomass production. Bioresour Technol 271:59–65. https://doi.org/10.1016/j.biortech.2018.09.082
Yang F, Xiang W, Fan J, Wu H, Li T, Long L (2016) High pH-induced flocculation of marine Chlorella sp. for biofuel production. J Appl Psychol 28:747–756. https://doi.org/10.1007/s10811-015-0576-7
Egloff S, Tschudi F, Schmautz Z, Refardt D (2018) High-density cultivation of microalgae continuously fed with unfiltered water from a recirculating aquaculture system. Microalgae Res 34:68–74. https://doi.org/10.1016/j.algal.2018.07.004
Zeng X, Danquah MK, Zhang S, Zhang X, Wu M, Chen XD, Ng IS, Jiang K, Lu Y (2012) Autotrophic cultivation of Spirulina platensis for CO2 fixation and phycocyanin production. Chem Eng Process 183:192–197. https://doi.org/10.1016/j.cej.2011.12.062
Perez-garcia O, Escalante FME, Bashan LE, Bashan Y (2010) Heterotrophic cultures of microalgae: metabolism and potential products. Water Res 45:11–36. https://doi.org/10.1016/j.watres.2010.08.037
Barros MP, Marin DP, Bolin AP, Macedo RCS, Compoio TR, Fineto CJ, Guerra BA, Polotow TG, Vardaris C, Mattei R, Otton R (2012) Combined astaxanthin and fish oil supplementation improves glutathione-based redox balance in rat plasma and neutrophils. Chem Biol Interact 197:58–67. https://doi.org/10.1016/j.cbi.2012.03.005
Vardon DR, Sharma BK, Scott J, Yu G, Wang Z, Schideman L, Zhang Y, Strathmann T (2011) Chemical properties of biocrude oil from the hydrothermal liquefaction of Spirulina algae, swine manure, and digested anaerobic sludge. Bioresour Technol 102:8295–8303. https://doi.org/10.1016/j.biortech.2011.06.041
Nam K, Lee H, Heo SW, Chang YK, Han JI (2016) Cultivation of Chlorella vulgaris with swine wastewater and potential for microalgae biodiesel production. J Appl Psychol 29:1171–1178. https://doi.org/10.1007/s10811-016-0987-0
Guihéneuf F, Stengel D (2013) LC-PUFA-enriched oil production by microalgae: accumulation of lipid and triacylglycerols containing n-3 LC-PUFA is triggered by nitrogen limitation and inorganic carbon availability in the marine haptophyte Pavlova lutheri. Mar Drugs 11:4246–4266. https://doi.org/10.3390/md11114246
Santos C, Uebel LS, Costa SS, Miranda AL, Morais EG, Morais MG, Costa JAV, Nunes IL, Ferreira ES, Druzian JI (2018) Outdoor pilot-scale cultivation of Spirulina sp. LEB-18 in different geographic locations for evaluating its growth and chemical composition. Bioresour Technol 256:86–94. https://doi.org/10.1016/j.biortech.2018.01.149
Tripathi R, Singh J, Thakur IS (2015) Characterization of microalgae Scenedesmus sp. ISTGA1 for potential CO2 sequestration and biodiesel production. Renew. Energy 74:774–781. https://doi.org/10.1016/j.renene.2014.09.005
Resolution from Brazilian National Agency for Petroleum, Natural Gas and Biofuels (2008) http://www.anp.gov.br. Accessed November 2019
UNE-EN 14104 (2003). Fat and oil derivatives. Fatty acid methyl esters (FAME). Determination of acid value and cold filter plugging point
Arias-Penarands MT, Cristiani-Urbina E, Montes-Horcasitas C, Esparza-Garcia F, Torzillo G, Canizares-Villanuera RO (2013) Scenedesmus incrassatulus CLHE-Si01: a potential source of renewable lipid for high quality biodiesel production. Bioresour Technol 140:158–164. https://doi.org/10.1016/j.biortech.2013.04.080
Jawaharraj K, Karpagam R, Ashokkumar B, Pratheeba CN, Varalakshmi P (2016) Enhancement of biodiesel potential in cyanobacteria: using agroindustrial wastes for fuel production, properties and acetyl CoA carboxylase D (accD) gene expression of Synechocystis sp.NN. Renew Energy 98:72–77. https://doi.org/10.1016/j.renene.2016.02.038
Sumprasit N, Wagle N, Glanpracha N, Annachhatre AP (2017) Biodiesel and biogas recovery from Spirulina platensis. Int Biodeterior Biodegradation 119:196–204. https://doi.org/10.1016/j.ibiod.2016.11.006
Francisco EC, Neves DB, Jacob-Lopes E, Franco TT (2010) Microalgae as feedstock for biodiesel production: carbon dioxide sequestration, lipid production and biofuel quality. J Chem Technol Biotechnol 85:395–403. https://doi.org/10.1002/jctb.2338
Knothe G (2005) Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process Technol 86:1059–1070. https://doi.org/10.1016/j.fuproc.2004.11.002
Knothe GH (2006) Some aspects of biodiesel oxidative stability. Fuel Process Technol 88:669–677. https://doi.org/10.1016/j.fuproc.2007.01.005
Deshmukha S, Kumar S, Bala K (2019) Microalgae biodiesel: a review on oil extraction, fatty acid composition, properties and effect on engine performance and emissions. Fuel Process Technol 191:232–247. https://doi.org/10.1016/j.fuproc.2019.03.013
Funding
This study was supported by the FAPESB—Foundation for Research Support of Bahia to project CNPQ (400710/2014-5) and by the MCTIC (Ministry of Technological Information and Communication Science)—Brazil and Bahia Pesca.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Spirulina sp. LEB 18 showed maximum biomass concentration in 100% wastewater aquaculture with 25% of Zarrouk (1.10 g L−1);
• Biomass production with higher values of protein (65.73%) and phycocyanin (16.60 mg/mL);
• High levels of polyunsaturated fatty acids (38.20%) and C18:3n6 (38.20%);
• High removal rates 94.01% (sulfate s); 93.84% (Phosphate); 96.77% (Bromine) and 90.00% (COD);
• T-25 treatment with quality biodiesel properties.
Rights and permissions
About this article
Cite this article
Cardoso, L.G., Duarte, J.H., Costa, J.A.V. et al. Spirulina sp. as a Bioremediation Agent for Aquaculture Wastewater: Production of High Added Value Compounds and Estimation of Theoretical Biodiesel. Bioenerg. Res. 14, 254–264 (2021). https://doi.org/10.1007/s12155-020-10153-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-020-10153-4