Abstract
Objective
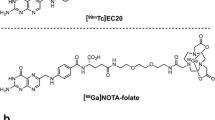
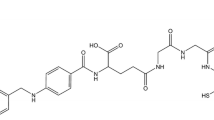
Technetium etarfolatide (99mTc-EF) is a radioactive diagnostic imaging agent that was developed to assess the expression of folate receptors in tumors. Administering folic acid prior to the administration of 99mTc-EF has been shown to improve SPECT images. Here, we conducted a phase I clinical trial to assess the safety, pharmacokinetics, and radiation dosimetry of 99mTc-EF injection following pre-administration of folic acid in healthy Japanese male adults.
Methods
Six healthy Japanese male adults were enrolled in the study. Folic acid was intravenously administered, followed 1–3 min later by an intravenous injection of 99mTc-EF (740 MBq ± 20 %). Assessments of subjective symptoms and objective findings, electrocardiograms, physical examination, and laboratory tests were performed before and up to 7 days after the injection to assess the safety of 99mTc-EF. Blood and urine collections and whole-body planar imaging were conducted at various time points up to 24 h after the injection to assess the pharmacokinetics of 99mTc-EF. The internal radiation dosimetry was calculated based on the pharmacokinetics results using the MIRD method.
Results
Five adverse events were observed in three subjects (50 %) after administration of the folic acid and 99mTc-EF, while these events were mild and non-serious. Of those five events, three were considered to be related to the administered agents. The radioactivity in blood rapidly decreased and showed a biphasic profile. The activity of 99mTc-EF at 5 min post injection was largest in the bone marrow, followed by the liver and kidneys, and had decreased within 24 h in all organs/tissues without appreciable retention. The pharmacokinetics results suggested that 99mTc-EF was mainly eliminated by kidney. The results also suggested that when administered at 925 MBq of 99mTc-EF, which is the maximum dose generally used for clinical trials in other countries, the corresponding effective dose of 99mTc-EF is equal to or less than those determined for the current radioactive diagnostic imaging agents.
Conclusions
The results of this study assessing the safety and radiation dosimetry of 99mTc-EF with folic acid pre-administration suggested that folic acid and 99mTc-EF should be appropriate for further studies. No pharmacokinetics concerns were noted.





Similar content being viewed by others
References
Ministry of Health, Labour and Welfare. Trends in leading causes of death. In: Summary of vital statistics, http://www.mhlw.go.jp/english/database/db-hw/populate/dl/03.pdf. Accessed 11 March 2015.
Sausville EA, Longo DL. Principles of cancer treatment. In: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, editors. Harrison’s principles of internal medicine. 18th ed. New York: McGraw-Hill; 2011. p. 705–6.
Toffoli G, Cernigoi C, Russo A, Gallo A, Bagnoli M, Boiocchi M. Overexpression of folate binding protein in ovarian cancers. Int J Cancer. 1997;74:193–8.
Bueno R, Appasani K, Mercer H, Lester S, Sugarbaker D. The α folate receptor is highly activated in malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 2001;121:225–33.
Ross JF, Chaudhuri PK, Ratnam M. Differential regulation of folate receptor isoforms in normal and malignant tissues in vivo and in established cell lines. Cancer. 1994;73:2432–43.
Weitman SD, Frazier KM, Kamen BA. The folate receptor in central nervous system malignancies of childhood. J Neurooncol. 1994;21:107–12.
Wu M, Gunning W, Ratnam M. Expression of folate receptor type α in relation to cell type, malignancy, and differentiation in ovary, uterus, and cervix. Cancer Epidemiol Biomarkers Prev. 1999;8:775–82.
Parker N, Turk MJ, Westrick E, Lewis JD, Low PS, Leamon CP. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal Biochem. 2005;338:284–93.
Reddy JA, Westrick E, Vlahov I, Howard SJ, Santhapuram HK, Leamon CP. Folate receptor specific anti-tumor activity of folate-mitomycin conjugates. Cancer Chemother Pharmacol. 2006;58:229–36.
Leamon CP, Reddy JA, Vlahov IR, Westrick E, Dawson A, Dorton R, et al. Preclinical antitumor activity of a novel folate-targeted dual drug conjugate. Mol Pharm. 2007;4:659–67.
Reddy JA, Dorton R, Westrick E, Dawson A, Smith T, Xu LC, et al. Preclinical evaluation of EC145, a folate-vinca alkaloid conjugate. Cancer Res. 2007;67:4434–42.
Leamon CP, Parker MA, Vlahov IR, Xu LC, Reddy JA, Vetzel M, et al. Synthesis and biological evaluation of EC20: a new folate-derived, 99mTc-Based radiopharmaceutical. Bioconjugate Chem. 2002;13:1200–10.
Fisher RE, Siegel BA, Edell SL, Oyesiku NM, Morgenstern DE, Messmann RA, et al. Exploratory study of 99mTc-EC20 imaging for identifying patients with folate receptor-positive solid tumors. J Nucl Med. 2008;49:899–906.
Maurer AH, Elsinga P, Fanti S, Nguyen B, Oyen WJG, Weber WA. Imaging the folate receptor on cancer cells with 99mTc-etarfolatide: properties, clinical use, and future potential of folate receptor imaging. J Nucl Med. 2014;55:701–4.
Endocyte. Safety and biodistribution of technetium Tc 99m EC20 in normal volunteers and ovarian cancer patients. https://clinicaltrials.gov/ct2/show/NCT01689636. Accessed 26 Dec 2014.
Endocyte. Evaluation of the biodistribution and safety of 99mTC-etarfolatide (EC20) in normal volunteers. https://clinicaltrials.gov/ct2/show/NCT01748864. Accessed 26 Dec 2014.
Endocyte. Safety and efficacy of FolateScan (technetium Tc 99m EC20) in women with suspected ovarian or endometrial cancer. https://clinicaltrials.gov/ct2/show/NCT01686256. Accessed 26 Dec 2014.
Endocyte. Study of EC0225 for the treatment of refractory or metastatic tumors. https://clinicaltrials.gov/ct2/show/NCT00441870. Accessed 26 Dec 2014.
Endocyte. Study of EC0489 for the treatment of refractory or metastatic tumors. https://clinicaltrials.gov/ct2/show/NCT00852189. Accessed 26 Dec 2014.
Merck Sharp and Dohme Corp. A study of MK-8109 (vintafolide) given alone or with chemotherapy in participants with advanced cancers (MK-8109-001). https://clinicaltrials.gov/ct2/show/NCT01688791. Accessed 26 Dec 2014.
Endocyte. Folic acid-tubulysin conjugate EC1456 in patients with advanced solid tumors. https://clinicaltrials.gov/ct2/show/NCT01999738. Accessed 26 Dec 2014.
Morris RT, Joyrich RN, Naumann RW, Shah NP, Maurer AH, Strauss HW, et al. Phase II study of treatment of advanced ovarian cancer with folate-receptor-targeted therapeutic (vintafolide) and companion SPECT-based imaging agent (99mTc-etarfolatide). Ann Oncol. 2014;25:852–8.
Endocyte. A phase II study of EC17 (folate-hapten conjugate) in patients with progressive metastatic renal cell carcinoma. https://clinicaltrials.gov/ct2/show/NCT00485563. Accessed 26 Dec 2014.
Merck Sharp and Dohme Corp. Study of vintafolide (MK-8109, EC145) in participants with progressive adenocarcinoma of the lung (MK-8109-008, EC-FV-03). https://clinicaltrials.gov/ct2/show/NCT00511485. Accessed 26 Dec 2014.
Naumann RW, Coleman RL, Burger RA, Sausville EA, Kutarska E, Ghamande SA, et al. PRECEDENT: a randomized phase II trial comparing vintafolide (EC145) and pegylated liposomal doxorubicin (PLD) in combination versus PLD alone in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2013;31:4400–6.
Endocyte. Phase 2 study of EC145 alone versus EC145 + Docetaxel versus Docetaxel alone in participants with FR(++) 2nd line non-small cell lung cancer (TARGET). https://clinicaltrials.gov/ct2/show/NCT01577654. Accessed 26 Dec 2014.
Endocyte. Study for women with platinum resistant ovarian cancer evaluating EC145 in combination with Doxil (PROCEED). https://clinicaltrials.gov/ct2/show/NCT01170650. Accessed 26 Dec 2014.
Endocyte. Investigator’s brochure of 99mTc-etarfolatide (EC20). Version 18.0. 2014.
ICRP. Radiation dose to patients from radiopharmaceuticals (Addendum to ICRP publication 53). ICRP Publication 80. Ann ICRP 1998;28(3).
ICRP, Radiological protection in biomedical research. ICRP publication 62. Ann ICRP 1992;22(3).
U.S. Food and Drug Administration. Section 361.1: Radioactive drugs for certain research use. In: Code of Federal Regulations Title 21: Food and Drugs, Part 361: Prescription drugs for human use generally recognized as safe and effective and not misbranded: drugs used in research. Code of Federal Regulations revised 1 Apr 2014; vol. 5.
Acknowledgments
We thank Dr. Yasushi Takagi of Showa University and Dr. Ukihide Tateishi of Tokyo Medical and Dental University for their support in the study design and data interpretation. We also thank the subjects as well as the staff of the Biomedical Research Center and the Department of Radiology at Kitasato University Kitasato Institute Hospital for their participation in the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This study was sponsored by Nihon Medi-Physics Co., Ltd (Tokyo, Japan). Pharma International Co., Ltd (Tokyo, Japan) supported the preparation of the article and the cost of the preparation was borne by Nihon Medi-Physics. Masahiro Omote is an employee of Nihon Medi-Physics.
Rights and permissions
About this article
Cite this article
Yamada, Y., Nakatani, H., Yanaihara, H. et al. Phase I clinical trial of 99mTc-etarfolatide, an imaging agent for folate receptor in healthy Japanese adults. Ann Nucl Med 29, 792–798 (2015). https://doi.org/10.1007/s12149-015-1006-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-015-1006-2