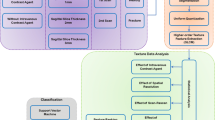
Abstract
Objective
The purpose is to develop and evaluate the ability of the computer-aided diagnosis (CAD) methods that apply texture analysis and pattern classification to differentiate malignant and benign bone and soft-tissue lesions on 18F-fluorodeoxy-glucose positron emission tomography/computed tomography (18F-FDG PET/CT) images.
Methods
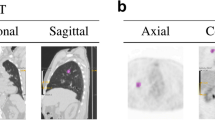
Subjects were 103 patients with 59 malignant and 44 benign bone and soft tissue lesions larger than 25 mm in diameter. Variable texture parameters of standardized uptake values (SUV) and CT Hounsfield unit values were three-dimensionally calculated in lesional volumes-of-interest segmented on PET/CT images. After selection of a subset of the most optimal texture parameters, a support vector machine classifier was used to automatically differentiate malignant and benign lesions. We developed three kinds of CAD method. Two of them utilized only texture parameters calculated on either CT or PET images, and the other one adopted the combined PET and CT texture parameters. Their abilities of differential diagnosis were compared with the SUV method with an optimal cut-off value of the maximum SUV.
Results
The CAD methods utilizing only optimal PET (or CT) texture parameters showed sensitivity of 83.05 % (81.35 %), specificity of 63.63 % (61.36 %), and accuracy of 74.76 % (72.82 %). Although the ability of differential diagnosis by PET or CT texture analysis alone was not significantly different from the SUV method whose sensitivity, specificity, and accuracy were 64.41, 61.36, and 63.11 % (the optimal cut-off SUVmax was 5.4 ± 0.9 in the 10-fold cross-validation test), the CAD method with the combined PET and CT optimal texture parameters (PET: entropy and coarseness, CT: entropy and correlation) exhibited significantly better performance compared with the SUV method (p = 0.0008), showing a sensitivity of 86.44 %, specificity of 77.27 %, and accuracy of 82.52 %.
Conclusions
The present CAD method using texture analysis to analyze the distribution/heterogeneity of SUV and CT values for malignant and benign bone and soft-tissue lesions improved the differential diagnosis on 18F-FDG PET/CT images.



Similar content being viewed by others
Notes
We define the length of the longest edge of the lesional bounding box as the diameter, because a lesion is not always circular.
We specify the developed CAD method to be the method utilizing the combination of CT and PET texture parameters in the following context.
References
Bischoff M, Bischoff G, Buck A, von Baer A, Pauls S, Scheffold F, et al. Integrated FDG-PET-CT: its role in the assessment of bone and soft tissue tumors. Arch Orthop Trauma Surg. 2010;130:819–27.
Charest M, Hickeson M, Lisbona R, Novales-Diaz JA, Derbekyan V, Turcotte RE. FDG PET/CT imaging in primary osseous and soft tissue sarcomas: a retrospective review of 212 cases. Eur J Nucl Med Mol Imaging. 2009;36:1944–51.
Piperkova E, Mikhaeil M, Mousavi A, Libes R, Viejo-Rullan F, Lin H, et al. Impact of PET and CT in PET/CT studies for staging and evaluating treatment response in bone and soft tissue sarcomas. Clin Nucl Med. 2009;34:146–50.
Aoki J, Endo K, Watanabe H, Shinozaki T, Yanagawa T, Ahmed AR, et al. FDG-PET for evaluating musculoskeletal tumors: a review. J Orthop Sci. 2003;8:435–41.
Schwarzbach MH, Dimitrakopoulou-Strauss A, Willeke F, Hinz U, Strauss LG, Zhang YM, et al. Clinical value of [18-F] fluorodeoxyglucose positron emission tomography imaging in soft tissue sarcomas. Ann Surg. 2000;231:380–6.
Shin DS, Shon OJ, Han DS, Choi JH, Chun KA, Cho IH. The clinical efficacy of 18F-FDG-PET/CT in benign and malignant musculoskeletal tumors. Ann Nucl Med. 2008;22:603–9.
Suga K, Chagawa K, Seki N, Taguchi T, Matsunaga N. Feasibility and pitfall of PET/CT scan in assessment of bone and soft-tissue tumors: initial experiences and literature review [in Japanese]. Jpn J Clin Radiol. 2011;56:855–72.
Dimitrakopoulou-Strauss A, Strauss LG, Heichel T, Wu H, Burger C, Bernd L, et al. The role of quantitative 18F-FDG PET studies for the differentiation of malignant and benign bone lesions. J Nucl Med. 2002;43:510–8.
Tixier F, Le Rest CC, Hatt M, Albarghach N, Pradier O, Metges JP, et al. Intratumor heterogeneity characterized by textural features on baseline 18F-FDG PET images predicts response to concomitant radiochemotherapy in esophageal cancer. J Nucl Med. 2011;52:369–78.
Tixier F, Hatt M, Le Rest CC, Le Pogam A, Corcos L, Visvikis D. Reproducibility of tumor uptake heterogeneity characterization through texture feature analysis in 18F-FDG PET. J Nucl Med. 2012;53:693–700.
Cook GJ, Yip C, Siddique M, Goh V, Chicklore S, Roy A, et al. Are pretreatment 18F-FDG PET tumor textural features in non-small cell lung cancer associated with response and survival after chemoradiotherapy? J Nucl Med. 2013;54:19–26.
Chicklore S, Goh V, Siddique M, Roy A, Marsden PK, Cook GJ. Quantifying tumour heterogeneity in 18F-FDG PET/CT imaging by texture analysis. Eur J Nucl Med Mol Imaging. 2013;40:133–40.
Davnall F, Yip CS, Ljungqvist G, Selmi M, Ng F, Sanghera B, et al. Assessment of tumor heterogeneity: an emerging imaging tool for clinical practice? Insights Imaging. 2012;3:573–89.
Haralick RM, Shanmugam K, Dinstein I. Textural features for image classification. IEEE Trans Syst Man Cybern. 1973;3:610–21.
Kurani AS, Xu DH, Furst J, Raicu DS. Co-occurrence matrices for volumetric data. In: Proceedings of 7th IASTED international conference on computer graphics and imaging; 2004.
Amadasun M, King R. Textural features corresponding to textural properties. IEEE Trans Syst Man Cybern. 1989;19:1264–74.
Kido S, Kuriyama K, Higashiyama M, Kasugai T, Kuroda C. Fractal analysis of small peripheral pulmonary nodules in thin-section CT: evaluation of the lung-nodule interfaces. J Comput Assist Tomogr. 2002;26:573–8.
Chen W, Giger ML, Li H, Bick U, Newstead GM. Volumetric texture analysis of breast lesions on contrast-enhanced magnetic resonance breast image data. J Magn Reson Imaging. 2007;58:562–71.
Brooks FJ, Grigsby PW. The effect of small tumor volumes on studies of intratumoral heterogeneity of tracer uptake. J Nucl Med. 2014;55:37–42.
Nestle U, Kremp S, Schaefer-Schuler A, Sebastian-Welsch C, Hellwig D, Rube C, et al. Comparison of different methods for delineation of 18F-FDG PET-positive tissue for target volume definition in radiotherapy of patients with non-small cell lung cancer. J Nucl Med. 2005;46:1342–8.
Tylski P, Stute S, Grotus N, Doyeux K, Hapdey S, Gardin I, et al. Comparative assessment of methods for estimating tumor volume and standardized uptake value in 18F-FDG PET. J Nucl Med. 2010;51:268–76.
Vapnik VN. The nature of statistical learning theory. 2nd ed. New York: Springer; 1999.
Chang CC, Lin CJ. LIBSVM: a library for support vector machines. ACM Trans Intell Syst Technol. 2011;2:27:1–27.
Japkowicz N, Shah M. Evaluating learning algorithms: a classification perspective. Cambridge: Cambridge University Press; 2011.
Fawceet T. An introduction to ROC analysis. Pattern Recogn Lett. 2006;27:861–74.
Shannon CE. A Mathematical Theory of communication. Bell Syst Tech J. 1948;27(379–423):623–56.
Meyer D, Leisch F, Hornik K. The support vector machine under test. Neurocomputing. 2003;55:169–86.
Acknowledgments
This research was supported in part by MEXT KAKENHI Grant Number 21103008, the MEXT-supported program for the strategic research foundation at private universities (2013–2017) and the R-GIRO research fund from Ritsumeikan University.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
The texture is defined as the spatial interrelationship between and/or spatial arrangement of FDG uptake (or HU values) inside target lesions. Texture analysis is a set of mathematical methods that extract such information by some quantified parameters. We utilized three statistics-based texture analysis methods, which were the gray-level histogram analysis (GLHA) based method, the spatial gray-level dependence matrix (SGLDM) based method [14, 15] and the neighborhood gray-tone difference matrices (NGTDM) based method [16].
GLHA parameters
GLHA parameters include 4 statistics (mean, standard deviation, skewness, and kurtosis) calculated from gray-level distributions (histogram) of voxels. Since these parameters are directly derived from a histogram (Fig. 2a), they can only reveal how the gray levels of voxels statistically vary without considering spatial relationships.
SGLDM parameters
SGLDM parameters are calculated from co-occurrence matrix describing how often a voxel of gray-level i finds a neighbor voxel with gray-level j according to a certain spatial relationship (Fig. 2b), so they are related to spatial relationships of a pair of consecutive voxels [14]. There are 13 co-occurrence matrices in 3D by considering 13 different angular directions (26-connexity) and 1-distance relationship between consecutive voxels [15]. On these matrices, ten parameters (Table 2) characterizing heterogeneity are calculated by averaging values, respectively, derived from these 13 matrices. Equations 1 and 2 define entropy and correlation, respectively.
where M 1(i, j) is a co-occurrence matrix, u x , u y , σ x, and σ y are the means and standard derivations of M x = ∑ j M 1(i, j) and M y = ∑ i M 1(i, j), respectively. The definitions of the other eight parameters can be referred to in the literature [14, 15].
NGTDM parameters
NGTDM parameters contain information about how the gray level of a voxel varies from all its neighbors (Fig. 2c). They are calculated from neighborhood gray-tone difference matrix M 2(i), whose entry describes the differences between voxels having gray-level i and their neighbors (26 neighbors in 3D). Five NGTDM parameters (Table 1) are calculated from the neighborhood gray-tone difference matrix. For example, Eq. 3 gives the definition of coarseness.
where p(i) is the probability of gray-level i existing inside the lesion, and ɛ is a small number to prevent coarseness becoming infinite. The definitions of the other four NGTDM can be referred to in the literature [16].
The units of texture parameters
All of the 19 parameters are statistical measures, and their units can be derived from the equations of their definitions. According to these definitions, each parameter is calculated from two kinds of term that are related to the intensity and probability of the intensity. Because the probability is a dimensionless measure, the units of the texture parameters are derived only from the voxel intensity. The SUV value is a dimensionless measure; however, the CT value is expressed in Hounsfield units (HU). Therefore, the same texture parameter that is calculated on PET or CT images has different units.
Since the SUV value does not have a unit, none of the texture parameters that are calculated on PET images have units either. For texture parameters calculated on CT images, some of them have units and the others do not. We summarize their units as follows: 7 dimensionless measures (skewness and kurtosis of the GLHA parameters; entropy, angular second moment, correlation and maximum probability of the SGLDM parameters; busyness of the NGTDM parameters), 4 measures with the unit of HU (mean and standard deviation of the GLHA parameters; sum mean of the SGLDM parameters; strength of the NGTDM parameters), 4 measures with the unit of HU2 (contrast, variance and cluster tendency of the SGLDM parameters; complexity of the NGTDM parameters), 1 measure with the unit of HU3 (contrast of the NGTDM parameters), 2 measures with the unit of 1/HU (homogeneity of the SGLDM parameters; coarseness of the NGTDM parameters) and 1 measure with the unit of 1/HU2 (inverse difference moment of the SGLDM parameters).
Rights and permissions
About this article
Cite this article
Xu, R., Kido, S., Suga, K. et al. Texture analysis on 18F-FDG PET/CT images to differentiate malignant and benign bone and soft-tissue lesions. Ann Nucl Med 28, 926–935 (2014). https://doi.org/10.1007/s12149-014-0895-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-014-0895-9