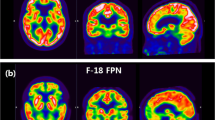
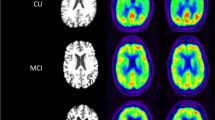
Abstract
Objective
To determine the optimal accumulation time for three-dimensional positron emission tomography (3D-PET) with 18F-2-fluoro-2-deoxy-d-glucose (18F-FDG) to detect the brain uptake pattern typical of Alzheimer’s disease (AD).
Methods
Patients with mild AD or amnestic mild cognitive impairment (MCI) and normal control subjects were recruited in the Japanese Alzheimer’s disease neuroimaging initiative and examined with a PET scan during the 30–60 min after FDG injection. Three independent blinded experts interpreted the 30- to 60-min sum images, and images of patients with AD and MCI presenting AD patterns and normal subjects presenting normal patterns were used in the analysis. Early-scan (ES) and late-scan (LS) images were obtained from the data acquired at 30–35 min and 55–60 min after the injection, respectively. Separate target regions of interest (ROI) for ES and LS were defined as areas of significant reductions in the posterior cingulate and parietotemporal lobe in both hemispheres from the results of an initial cohort with 21 patients (AD 16, MCI 5) and 19 controls. A subsequent sample of 36 (AD 9, MCI 27) patients and 38 controls were used to compare the diagnostic capability of ES and LS using Z scores within the target ROI in individual statistical parametric mapping analysis.
Results
Compared to LS, ES showed lower activity in the frontal lobes and higher activity in the venous sinus than LS; however, the diagnostic capability of ES and LS did not significantly differ (sensitivity 0.97 and 0.97, specificity 0.82 and 0.84, area under the receiver-operating characteristic curve 0.96 and 0.97, respectively).
Conclusions
For a qualitative diagnosis of the AD pattern in 3D FDG-PET, results of ES were equivalent to those of LS. ES may be an option to shorten the entire PET procedure time, particularly in diagnosing early stages of AD.




Similar content being viewed by others
References
Silverman DH, Small GW, Chang CY, Lu CS, Kung De Aburto MA, Chen W, et al. Positron emission tomography in evaluation of dementia: regional brain metabolism and long-term outcome. JAMA. 2001;286:2120–7.
Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol. 1997;42:85–94.
Phelps ME, Huang SC, Hoffman EJ, Selin C, Sokoloff L, Kuhl DE. Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18)2-fluoro-2-deoxy-d-glucose: validation of method. Ann Neurol. 1979;6:371–88.
Lucignani G, Schmidt KC, Moresco RM, Striano G, Colombo F, Sokoloff L, et al. Measurement of regional cerebral glucose utilization with fluorine-18-FDG and PET in heterogeneous tissues: theoretical considerations and practical procedure. J Nucl Med. 1993;34:360–9.
Schmidt KC, Lucignani G, Sokoloff L. Fluorine-18-fluorodeoxyglucose PET to determine regional cerebral glucose utilization: a re-examination. J Nucl Med. 1996;37:394–9.
Sakamoto S, Ishii K, Hosaka K, Mori T, Sasaki M, Mori E. Detectability of hypometabolic regions in mild Alzheimer disease: function of time after the injection of 2-[fluorine 18]-fluoro-2-deoxy-d-glucose. AJNR Am J Neuroradiol. 2005;26:843–7.
Chen WP, Matsunari I, Noda A, Yanase D, Yajima K, Takeda N, et al. Rapid scanning protocol for brain (18)F-FDG PET: a validation study. J Nucl Med. 2005;46:1633–41.
Arai H, Okamura N, Furukawa K, Kudo Y. Geriatric medicine, Japanese Alzheimer’s disease neuroimaging initiative and biomarker development. Tohoku J Exp Med. 2010;221:87–95.
Ikari Y, Nishio T, Makishi Y, Miya Y, Ito K, Koeppe RA, et al. Head motion evaluation and correction for PET scans with 18F-FDG in the Japanese Alzheimer’s disease neuroimaging initiative (J-ADNI) multi-center study. Ann Nucl Med. 2012;26:535–44.
Cross DJ, Minoshima S, Nishimura S, Noda A, Tsukada H, Kuhl DE. Three-dimensional stereotactic surface projection analysis of macaque brain PET: development and initial applications. J Nucl Med. 2000;41:1879–87.
Arnaiz E, Jelic V, Almkvist O, Wahlund LO, Winblad B, Valind S, et al. Impaired cerebral glucose metabolism and cognitive functioning predict deterioration in mild cognitive impairment. NeuroReport. 2001;12:851–5.
Herholz K, Salmon E, Perani D, Baron JC, Holthoff V, Frolich L, et al. Discrimination between Alzheimer dementia and controls by automated analysis of multicenter FDG PET. Neuroimage. 2002;17:302–16.
Drzezga A, Lautenschlager N, Siebner H, Riemenschneider M, Willoch F, Minoshima S, et al. Cerebral metabolic changes accompanying conversion of mild cognitive impairment into Alzheimer’s disease: a PET follow-up study. Eur J Nucl Med Mol Imaging. 2003;30:1104–13.
Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NU, Wenger KK, et al. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. Neuroimage. 2007;35:396–405.
Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–82.
Acknowledgments
This study is a part of the “Translational Research Promotion Project/Research project for the development of a systematic method for the assessment of Alzheimer’s disease,” sponsored by the New Energy and Industrial Technology Development Organization (NEDO) of Japan. J-ADNI is also supported by a Grant-in-Aid for Comprehensive Research on Dementia from the Japanese Ministry of Health, Labour and Welfare, as well as by the grants from J-ADNI Pharmaceutical Industry Scientific Advisory Board (ISAB) companies. The authors would also like to thank the J-ADNI Imaging ISAB and other organizations for their support of this work.
Conflict of interest
The authors report no conflict of interest.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Takahashi, R., Ishii, K., Senda, M. et al. Equal sensitivity of early and late scans after injection of FDG for the detection of Alzheimer pattern: an analysis of 3D PET data from J-ADNI, a multi-center study. Ann Nucl Med 27, 452–459 (2013). https://doi.org/10.1007/s12149-013-0704-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-013-0704-x