Abstract
Motivation plays a critical role in human cognitive function, while acting as a driving force for the necessary behavior to achieve a desired goal and success (i.e., achievement motivation). Based on the theoretical background of achievement motivation, this study designed an incentive delay task with four motivational orientations (i.e., promotion, prevention, mastery/self, and performance/other). To investigate whether people would have their behavioral patterns toward achievement motivation orientation, we applied an unsupervised clustering algorithm to classify individuals’ behavioral responses acquired from the task by categorizing certain behavioral similarities. As a result, this hierarchical clustering approach classified subjects into two distinctive subgroups: Group#1 (i.e., the pro/pre group, n = 52) and Group#2 (i.e., the self/other group, n = 48). Based on clustering, Group#1 showed significantly better performance with promotion/prevention orientations, whereas Group#2 exhibited significantly higher performance with self/other orientations. Structural brain analyses discovered increased gray matter volume and sulcal depth in the posterior parietal cortex (PPC) in the pro/pre group compared to the self/other group. With resting-state functional magnetic resonance imaging data, we found higher local brain fluctuations in the medial prefrontal cortex (mPFC) in the self/other group compared to the pro/pre group. Furthermore, mPFC seed-based functional connectivity showed significantly increased functional coupling with the posterior cingulate cortex in the self/other group relative to the pro/pre group. Taken together, these results shed light on structural and functional neural mechanisms related to achievement motivation and, furthermore, provide novel insights regarding PPC’s role in motivational processing toward promotion- and prevention-focused orientation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Motivation plays a crucial role in human behaviors such as decision making, goal setting, academic and career success (Bryan & Locke, 1967; Maddox & Markman, 2010). Motivation is the force that drives one’s actions toward a desired goal (i.e., achievement motivation) (Murayama et al., 2012). The orientations of the achievement motivation are relatively stable personal dispositions that act as an important source for achieving a particular goal (Atkinson, 1957). The chosen achievement motivation orientation while pursuing a goal can account for comprehensive behavioral and neural differences (Ames, 1992; Kim et al., 2016). For example, achievement motivation orientation is related to positive sensitivity, and associated with stronger activations in reward-related brain regions (Elliot & Thrash, 2010; Swanson & Tricomi, 2014), whereas a motivation deficiency has been linked to emotional vulnerabilities such as depression (Strauman & Eddington, 2017).
The construct of achievement motivation has occurred through the combined theoretical and conceptual models. It was initially developed as two dichotomous models: the learning-performance and task-ego (Nicholls, 1989; Smiley & Dweck, 1994). These two dichotomous models were later unified into a single framework with two types of motivations: mastery and performance (Ames & Archer, 1988). Previous studies proved that these two types of motivational orientation were related to social comparison (Darnon et al., 2010; Park & Park, 2017; Régner et al., 2007). The social comparison dimension distinguishes motivational orientations based on whether one compares themselves with oneself or with others (hereafter referred to as “self/other dimension”), which can be mastery and performance goals (Ames, 1992; Elliot & McGregor, 2001; Midgley et al., 2000). Mastery and performance goals have different perspectives on success/achievement and involve different perceptions toward oneself and task performance. Mastery-oriented individuals are more focused on personal success and accomplishments and therefore strive to advance their skills or to gain new knowledge. Performance-oriented people are focused on superior performance compared to others and thus tend to seek social prestige (Ames, 1992).
Following the aforementioned motivational orientation, another framework of achievement motivation was introduced as approach-avoidance orientation (Elliot & Church, 1997; Elliot & McGregor, 2001; Elliot & Thrash, 2010). This corresponds to promotion-prevention motivational orientation, which has been extensively used as regulatory focus theory in the personal goal pursuit (Higgins, 1997). The regulatory focus dimension postulates two separate self-regulatory motivational orientations, promotion and prevention (hereafter referred to as “pro/pre dimension”), based on how individual motivation is evaluated, either by reaching gains or avoiding loss (Higgins, 1997). Promotion-focused individuals regulate themselves toward positive end-states with emphasis on eager aspirations and approach strategy. They view goals as ideals, hopes, accomplishments, and advancements. On the other hand, prevention-focused individuals employ avoidance-oriented strategies and focus on the absence of negative outcomes. They have goal perspectives as ought, and they concordantly prioritize duties, obligations, and responsibilities (Detloff et al., 2020; Eddington et al., 2007).
Previous neuroimaging studies have revealed the neural circuits associated with the abovementioned motivational orientations (Detloff et al., 2020; Eddington et al., 2007; Kim et al., 2016). The valuation circuit involves a value-based decision-making that estimates various options in the choice set, continuously evaluating and updating values. Understanding stimulus values, people can approach or avoid behaviors according to their goal orientations. This circuit includes the ventral striatum, ventromedial prefrontal cortex (vmPFC), medial orbitofrontal cortex (mOFC), and the precuneus and posterior parietal cortex (PPC) (Bartra et al., 2013; Jocham et al., 2014; Rangel et al., 2008). The default mode network (DMN) is involved in self-regulation during personal goal pursuit while internally focusing cognition and self-/other-referential thoughts (Badre & Nee, 2018; Detloff et al., 2020). This network consists of the medial prefrontal cortex (mPFC) and the posterior cingulate cortex (PCC), also found in the valuation circuit, as well as the precuneus, the lateral and medial temporal lobes, and the posterior inferior parietal lobule (Buckner et al., 2008). The salience network is engaged for motivationally salient stimuli and leads to approach behaviors that trigger reward. This network consists of the anterior cingulate cortex, anterior insula, amygdala, and dorsal striatum (Haber & Knutson, 2010; O’Doherty et al., 2003). Motivational orientation is generated by interactions among these neurocircuitries, through knowledge of stimulus values, self/other references, and incentive salience.
Though several studies have explored the neural underpinnings of a single achievement motivation dimension (i.e., either pro/pre or self/other dimensions), to our knowledge, no brain imaging study has yet investigated both simultaneously. Based on above mentioned theoretical backgrounds, we designed a task with four achievement motivation orientations (i.e., promotion, prevention, mastery/self, and performance/other) from two primary motivational dimensions (i.e., pro/pre and self/other dimensions). We used a logistic regression model to infer the degree of influence of each achievement motivation orientation on the individual’s behavioral performance. Next, we applied data clustering algorithms, which have been increasingly used in many different study fields for classification by maximizing a certain similarity condition (Antunes, 2021; Kou et al., 2014; Kou et al., 2020; Li & Xu, 2021). Using a hypothesis-free data-driven unsupervised clustering algorithm, we distinguished groups of individuals by behavioral similarities to examine group-specific behavioral characteristics and the involved neural networks. To integrate the behavioral measurements into neural evidence, we performed several whole-brain voxel-level analyses using voxel-based morphometry (VBM) and surface-based morphometry (SBM) to identify the neural differences in intrinsic structural architectures underlying each type of achievement motivation orientation. To further identify group-specific functional networks in terms of the local spontaneous neural activity and functional connectivity (FC), we performed amplitude of low-frequency fluctuation (ALFF) and seed-based FC approaches to resting-state functional MRI (rsfMRI) data. Given the aforementioned networks that are associated with achievement motivation, we hypothesized that the pro/pre dimension would associate more with regions in the valuation and salience network, whereas the self/other dimension would relate more to regions belonging to the DMN.
Methods
Participants
All data in this study were collected as part of the Psychological and Neural Mechanisms for Predicting Academic Achievement study. For said study, participants were asked to fill out a series of questionnaires (such as their achievement goals, motivation, time perspectives, social orientation, self-construal, and personality traits), performed several choice behavioral tasks (including a modified incentive delay task, an intertemporal choice task, and a risk tolerance task), and underwent brain scans. The scanning session consisted of high-resolution T1-weighted anatomical MRI, rsfMRI, diffusion tensor imaging, and fMRI during cognitive tasks. All participants had normal or corrected-to-normal vision with no significant medical illness. In this study, we used data from the modified incentive delay task, T1-weighted MRI, and rsfMRI to examine anatomical and functional neural signatures of achievement motivation. All participants were young healthy adults that provided written informed consent before participation.
Of all participants (n = 115), 102 completed both the incentive delay task and brain scans. We excluded two of them due to either poor-quality brain images (n = 1) or as an outlier on behavioral performance (n = 1). Therefore, 100 participants (52/48 males/females; age [mean ± SD], 22.2 ± 2.88 years; the duration of education, 15.02 ± 1.29 years) were used for the final analysis.
Task
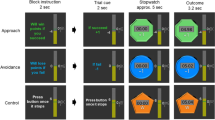
We used a modified version of the monetary incentive delay task (Fig. 1), developed by Knutson et al. (Knutson & Gibbs, 2007), in the context of achievement motivation orientations. The task consisted of five conditions: four derived from the combination of regulatory focus [i.e., promotion vs. prevention] and social comparison standard [i.e., self vs. other], along with one control condition not associated with any motivational dimension. For all conditions, each trial comprised four phases: cue presentation, anticipation, target presentation, and feedback. During the cue presentation phase, a cue indicating one of five conditions was presented: self-promotion (press a button faster than my reaction time (RT), that is, the average RT of accumulated behavioral responses while performing a task), self-prevention (press a button not slower than my RT), other-promotion (press a button faster than others’ RT, that is, the average RT of the subjects who participated in this study before me), other-prevention (press a button not slower than others’ RT), and control (no feedback). During the target presentation phase, participants had to press a button as fast as possible while each trial’s target was displayed. Then, a feedback was presented to subjects according to their responses. All participants completed a practice session of the task to understand how to complete it and to provide an estimate of each individual’s RT for standardizing the task difficulty; mean RT during the practice task was used as target duration in the first trial. To control the task difficulty, the target duration decreased 30 ms on the next trial after a correct response and increased 40 ms on the next trial after an incorrect response.
Task design. For all conditions, each trial comprised four phases: cue presentation, anticipation, target presentation, and feedback. During the cue presentation phase, a cue indicating one of five conditions was presented: self-promotion (press a button faster than myself), self-prevention (press a button not slower than myself), other-promotion (press a button faster than others), other-prevention (press a button not slower than others), and control (no feedback)
Behavioral model and hierarchical cluster analysis
To infer the degree of influence of each motivational feature on the individual’s hit/miss probability, we used a logistic regression model. In this model, five features were included to estimate the extent to which the dimensions or features affected individual’s response: two features each from pro/pre and self/other dimensions and difficulty levels. The difficulty levels modeled the current target durations, either increasing or decreasing based on whether the previous trial’s response was correct or not, and coded as follows: Dt+1 = Dt − 1 for an incorrect trial, Dt+1 = Dt +1 for a correct trial, and D0 = 0 for the first trial. The dependent variable y denotes each participant’s hits and misses, and the independent variable x denotes the 5 features. The beta coefficient β indicates how much individual’s hit/miss probability was modulated by each motivational feature and difficulty level. β0 represents the intercept, which is the performance rate in the control condition.
The model was implemented as below, and experimental variables were dummy coded, with “1” referring to each experimental variable, and “0” referring to all other conditions, to extract each feature for subsequent model-based brain imaging analyses.
Logit(p(y) = 1) = β0 + βproxpro + βprexpre + βselfxself + βotherxother + βdifficultyxdifficulty
To categorize groups according to their behavioral similarities, the β estimated from all participants was used to perform agglomerative hierarchical clustering analysis with MATLAB (release R2021b, the MathWorks, Inc., Natick, Massachusetts) as the individual weighting of each feature (βpro, βpre, βself, and βother) may vary in our motivational study paradigm. The hierarchical clustering analysis was based on Ward’s method to combine pairs of clusters at each step while minimizing the sum of square errors from the cluster mean. The Euclidean distance approach was used to measure the distance values. To find the optimal number of clusters, we used Mojena’s stopping rule (Mojena, 1977), a well-accepted rule for number of clusters (Milligan & Cooper, 1985).
Brain imaging
Image acquisition
All images were obtained using a 3-T scanner (Siemens Magnetom Trio; Erlangen, Germany). High-resolution T1-weighted anatomical images were acquired using a 3D magnetization-prepared, rapid-acquisition gradient echo sequence [repetition time (TR) = 1,900 ms, echo time (TE) = 2. 52 ms, flip angle (FA) = 9°, voxel size = 1.0 × 1.0 × 1.0mm, 192 sagittal slices]. For each participant, 155 functional images were acquired using T2*-weighted, echo-planar imaging (EPI; TR = 2,000 ms, TE = 20 ms, FA = 90°, voxel size = 3.0 × 3.0 × 3.0mm, 42 interleaved axial slices). Participants were asked to relax with their eyes open and maintain fixation during rsfMRI. An eye-tracker mounted on a head coil was used to monitor the participants’ eyes and ensure they did not fall asleep during the scan.
Structural data analysis
T1-weighted MRI scans are analyzed in both VBM and SBM approaches using the CAT12 toolbox (Department of Psychiatry, University of Jena, Jena, Germany; http://www.neuro.uni-jena.de/cat) within the SPM12 framework (http://www.fil.ion.ucl.ac.uk/spm/; Wellcome Department of Cognitive Neurology), using MATLAB R2021b (Mathworks). For VBM analyses, images were segmented into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) components, and then spatially registered using a CAT12’s default setting. All images were inspected for image quality and homogeneity. Images were smoothed with a Gaussian kernel of 8mm. For SBM analyses, we estimated both cortical thickness and sulcus depth; cortical thickness was defined as the distance between WM and GM voxels based on the projection-based thickness method (Dahnke et al., 2013) and the sulcus depth with square root function transformation was defined as the Euclidean distance between the central surface and the convex hull. Images were smoothed using a Gaussian kernel with a full-width-half-maximum (FWHM) of 20mm.
To determine differences in structural brain features between groups, a two-sample t-test was performed to compare the GM volume with age, sex, and total intracranial volume (TIV) as covariates. The same statistical analysis was conducted for each cortical thickness and sulcus depth data with age and sex as covariates. To correct for multiple comparisons, we used a threshold of voxel-wise P < 0.001 and cluster-level P < 0.05 family-wise error corrected (Eklund et al., 2016).
To confirm our findings of group-specific structural differences on motivational orientations, we implemented whole-brain multiple regression analyses to characterize the structural brain variables relevant to four β of interests (βpro, βpre, βself, and βother) in all groups. In these analyses, each structural image map along with all four βs (βpro, βpre, βself, and βother) were included and controlled for age, sex, and TIV as covariates in the VBM, and for age and sex in the SBM.
Functional data analysis
After discarding the first four volumes, fMRI data were preprocessed using SPM12 and DPARSFA toolbox (www.rfmri.org/DPARSF) (Chao-Gan & Yu-Feng, 2010). Preprocessing steps consisted of slice-acquisition timing, motion correction, nuisance signal regression, spatial normalization, spatial smoothing, and band-pass filtering. All data showed (i) six motion parameters < 1 voxel (2.5mm or 2.5°) in any direction and (ii) a mean frame-wise displacement (FD) < 0.30 (Power et al., 2012). To remove the effects of head motion and non-neuronal physiological signals, the following nuisance parameters were regressed out: Friston 24-motion parameters, five principal components estimated from the WM and CSF mask using the anatomical component-based noise correction method [aCompCor; (Behzadi et al., 2007)], and a linear detrending term. Afterwards, the residual images were spatially normalized to the MNI space, smoothed using a FWHM of 6-mm, and band-pass filtered (0.01–0.1Hz).
To investigate whether the morphological differences are also associated with the functional brain networks, we performed seed-based FC analysis using the region identified in VBM analysis as seed. That is, we defined a 5-mm radius spherical seed centered on the PPC [MNI, − 26 − 82 39] identified from the VBM analysis (see Results). Next, the Pearson correlation coefficients between the mean time series of the seed and that from all other voxels were calculated. The correlation coefficients were then Fisher r-to-z transformed. The two-sample t-test was performed to test the group differences from the whole-brain seed-based FC maps using age and sex as covariates.
To investigate regional differences in brain function, particularly in terms of low-frequency local fluctuation in brain activity between the two groups at the whole-brain voxel level, we further performed ALFF analysis using DPARSFA default settings. One-sample t-tests with these ALFF functional maps were performed to create mask images for between-group analyses to restrict significant voxels across individuals. Then, two-sample t-tests with the mask images were used to compare ALFF functional maps between groups.
Last, to explore whether the group differences in the local neural activity may be associated with differences in its FC, we performed the abovementioned seed-based FC analysis to the region (i.e., mPFC [MNI, − 6, 54, 3]; see Results) identified from ALFF analysis. Then, a two-sample t-test was conducted to identify between-group FC differences. The results of all analyses were obtained using a threshold of voxel-wise P < 0.001 and cluster-level P < 0.05 family-wise error corrected, consistent with structural analyses.
To examine the functional local neural activity characterized by each motivational orientation, we implemented whole-brain multiple regression analyses to examine the functional brain variables related to four β of interests (βpro, βpre, βself, and βother). In these analyses, each ALFF map along with all four βs (βpro, βpre, βself, and βother) were included while controlling for age and sex as covariates.
Results
Behavioral results
For hierarchical clustering of participants’ behavior data, we determined the optimal number of clusters to be two, of which distinct subgroups emerged with different patterns of behavioral responses (Online Resource 1). Based on clustering, Group#1 (i.e., the pro/pre group, n = 52) showed better performance in the pro/pre dimension, while Group#2 (i.e., the self/other group, n = 48) exhibited higher performance in the self/other dimension. A Mann–Whitney U-test, comparing the pro/pre group with the self/other group in each of five βs, confirmed that Group#1 had significantly better performance on the pro/pre dimension than Group#2 and that Group#2 had significantly better performance on the self/other dimension than Group#1 (ps < 0.001 in the βpro, βpre, βself, and βother between groups; p = 0.338 in the βdifficulty between groups; Fig. 2). There were no significant demographic differences in age (22.33 ± 3.21 for Group#1; 22.06 ± 2.50 for Group#2; p = 0.881) and sex (29 males and 23 females for Group#1; 23 males and 25 females for Group#2; p = 0.435) between the two groups.
Behavioral results of βs (βpro, βpre, βself, βother, and βdifficulty) from group comparison. The hierarchical clustering algorithm separates our data into two different subgroups, one for the pro/pre group (Group#1) and the other for the self/other group (Group#2). Group#1 showed significantly better performance in the pro/pre dimension, compared to the Group#2 (p < 0.001 in the βpro and βpre). Group#2 exhibited significantly higher performance than Group#1 (p < 0.001 in the βself and βother). The difficulty feature showed no significant group differences (p = 0.338 in the βdifficulty)
Structural brain imaging results
VBM analyses revealed that the left PPC GM volume was higher in Group#1 than in Group#2 (x, y, z coordinates = − 26, − 82, 39; z-value = 4.15; uncorrected significance level p < 0.001; FWE-corrected extent p < 0.05; Fig. 3a). We also found higher sulcus depth in the right PPC in Group#1 than in Group#2 (x, y, z coordinates = 23, − 63, 44; z-value = 4.44; uncorrected significance level p < 0.001; FWE-corrected extent p < 0.05; Fig. 3b), though in different brain hemispheres. There were no significant differences in cortical thickness maps between groups.
Further multiple regression analyses with four βs (βpro, βpre, βself, and βother) were conducted as confirmatory analysis. When examining βpro jointly with βpre, we found a positive correlation in the right PPC (x, y, z coordinates = 24, − 69, 33; z-value = 5.03; uncorrected significance level p < 0.001; FWE-corrected extent p < 0.05; Online Resource 2); the same region observed in the sulcus depth result. There were no significant correlations between any brain regions and self/other dimension.
VBM and SBM results. (a) The GM volume in the PPC significantly increased in the pro/pre group (x, y, z coordinates = − 26, − 82, 39; z-value = 4.15) (b) Deeper sulcal depth was observed in the pro/pre group compared to that in the self/other group (x, y, z coordinates = 23, − 63, 44; z-value = 4.44). Sulcal depth image was thresholded at p < 0.01 uncorrected for visualization purposes
Functional brain imaging results
We selected the above-identified left PPC region as seed and generated seed-based FC maps. Both groups exhibited significant FC between the PPC-seed and regions including the dorsolateral prefrontal cortex and anterior inferior parietal lobule, as well as the cuneus, fusiform gyrus, and lingual gyrus. However, there were no significant differences in PPC seed-based FC maps between groups.
To further examine whether there were group differences in terms of local neural activity at the whole-brain voxel-level, we performed an ALFF analysis. The two-sample t-test on the ALFF maps showed significantly higher ALFF values in the left mPFC region (x, y, z coordinates = − 6, 54, 3; z-value = 3.97; Fig. 4a) in Group#2 compared to Group#1. Furthermore, one-sample t-test using seed-based FC maps with the left mPFC region as a seed showed the DMN in both groups (Fig. 4b). Then, a two-sample t-test revealed increased mPFC seed-based FC with the PCC (x, y, z coordinates = 6, − 39, 15; z-value = 3.79; Fig. 4c) in Group#2 (vs. Group#1). This result verified that self/other-oriented individuals are susceptible to processing oneself and others information by demonstrating stronger FC between the mPFC and PCC in the DMN.
Further multiple regression analyses with four βs (βpro, βpre, βself, and βother) were conducted. βpro was positively correlated with the right OFC (x, y, z coordinates = 39, 51, − 9; z-value = 4.95; uncorrected significance level p < 0.001; FWE-corrected extent p < 0.05; Online Resource 3a), but negatively correlated with the right parietal cortex (x, y, z coordinates = 9, − 42, 69; z-value = 4.67; uncorrected significance level p < 0.001; FWE-corrected extent p < 0.05; Online Resource 3b). On the other hand, βpre showed a positive correlation with the right parietal cortex (x, y, z coordinates = 3, − 33, 69; z-value = 4.39; uncorrected significance level p < 0.001; FWE-corrected extent p < 0.05; Online Resource 3c) and right visual cortex (x, y, z coordinates = 6, − 90, 24; z-value = 4.25; uncorrected significance level p < 0.001; FWE-corrected extent p < 0.05; Online Resource 3d). However, βpre exhibited a negative correlation with the right OFC (x, y, z coordinates = 45, 45, − 6; z-value = 5.07; uncorrected significance level p < 0.001; FWE-corrected extent p < 0.05; Online Resource 3e). Notably, the right OFC and parietal cortex showed opposite patterns of correlations with the βpro and βpre, suggesting that the promotion- and prevention-focused orientations may differently relate to local FC within the frontal-parietal interaction.
Voxel-level, whole-brain rsfMRI analyses. (a) The whole-brain ALFF result exhibited higher left mPFC ALFF in the self/other group compared to that in the pro/pre group (x, y, z coordinates = − 6, 54, 3; z-value = 3.97). (b) Using the mPFC as seed region, one-sample t-test with ALFF functional maps served to create mask images for between-group analyses to restrict significant voxels across individuals. As a result, the DMN was observed in both groups (red for the self/other group; blue for the pro/pre group). (c) With the left mPFC as a seed region, the FC between the mPFC and PCC significantly increased in the self/other group relative to that in the pro/pre group (x, y, z coordinates = 6, − 39, 15; z-value = 3.79)
Discussion
In the current study, based on the theoretical and conceptual foundations of achievement motivation, we investigated whether behavioral characteristics were classified by a hypothesis-free, data-driven approach and furthermore, this classification would lead to neural differences. As results, we found that the agglomerative unsupervised clustering approach naturally classified our behavioral data into two subgroups according to behavioral patterns on achievement motivation orientations; these subgroups showed intrinsic group-specific structural and functional neural signatures. Compared to Group #2, Group#1 had better performance in promotion- and prevention-focused motivation and these motivational orientations were significantly associated with structural brain variables in the PPC, which belongs to the valuation network. This region thus serves as a neural predictor for promotion- and prevention-focused motivational processing, as confirmed by multiple regression analyses. However, compared to Group #1, Group#2 had better performance in self/other dimension and had significantly higher local brain fluctuations in the mPFC and increased FC between the mPFC and PCC, which belong to the DMN. Taken together, our findings indicate that the two different types of achievement motivation dimensions can be characterized by different neural structures and functional networks.
Applying a logistic regression model, we could examine four distinct motivational orientations derived from the two conceptualized dimensions. When we performed unsupervised hierarchical clustering on these four orientations, we found two subgroups naturally matching the original two dimensions. Our behavioral results indicate that each subgroup preferentially placed more attention on their preferred pro/pre or self/other dimension, and that this autonomic attentional priority eventually led to better performance in their respective conditions.
The present study showed higher GM volume of the PPC in the pro/pre group compared to that of the self/other group. This result can be interpreted according to valuation, since promotion and prevention goal types correspond to positive and negative value, respectively (Kahnt et al., 2014). Previous studies have shown that the PPC, as a member of the valuation network, represents value signal processing, as well as salience (Iyer et al., 2010; Jocham et al., 2014; Peck et al., 2009). Hence, individuals may have their own behavioral predispositions in their weighting of positive or negative values; these value-based behavioral patterns can be characterized by particular brain structural patterns. The PPC has also been associated with stimulus-driven attention such as perceptual salience and spatial attention, and it thus underlies numerous cognitive processes implying also cortical and subcortical regions (Corbetta et al., 2008; Theeuwes, 1994). Jocham and colleagues also discovered that this area was correlated with stimulus-driven decision-making processing in short time periods (Jocham et al., 2014). Promotion and prevention behavioral predispositions are more instantaneously aroused when people encounter informative stimuli (Elliot & McGregor, 2001). Therefore, the more instant decision-making process of promotion and prevention may be underpinned by the PPC’s neural signature.
In line with the VBM result, we observed deeper sulcal depth in the right PPC, although the brain lateralization was opposite of that obtained from the VBM analysis. Although the mechanism of sulcal depth is not yet been fully understood, this finding could imply that the increase of the GM volume and sulcal depth were related. Notably, as confirmed by multiple regression analyses, the same right PPC region was positively correlated with combined contrasts of βpro and βpre. Therefore, the structural increase in the PPC is predicted by individuals leaning more toward the pro/pre dimension. Our data extend the neural representation of individuals’ promotion and prevention tendencies into the parietal region, especially the PPC, by showing its neuroanatomical correlates.
In this study, seed-based FC using the left PPC as seed region showed neural interactions with the dorsolateral prefrontal cortex and anterior inferior parietal lobule, as well as the cuneus, fusiform gyrus, and lingual gyrus. However, these neural networks did not significantly differ between groups. This may mean that the PPC works with the prefrontal regions in both groups, extending its FC to neighboring neural regions including the cuneus, fusiform and lingual gyrus, suggesting underlying planning and visualizing for forthcoming actions during motivational goal pursuit (Detloff et al., 2020).
Furthermore, self/other oriented individuals exhibited increased ALFF in the mPFC; interestingly, the mPFC seed-based FC analysis revealed FC with the DMN in both groups. As a result of the group differences on mPFC seed-based FC maps, the self/other group, compared to the pro/pre group, showed increased FC between the mPFC and PCC, which are core regions in the DMN (Andrews-Hanna et al., 2014; Raichle et al., 2001). In rsfMRI data, the ALFF measures the amplitude of regional spontaneous neuronal activity (Yang et al., 2007; Zang et al., 2007), whereas the FC determines temporal synchrony between distant brain regions. The PCC is a heterogeneous area in functional terms and is involved in nearly all tasks that require self-generated processing. Similarly, the mPFC is engaged in decision-making process relevant to other people. Both the mPFC and PCC have an extensive pattern of connections and, as members of the DMN, a significant functional role inferring the thoughts and feelings of self and others. Therefore, our finding suggests that individuals who tend to be more sensitive to self and others have higher intrinsic local functional activity in the mPFC and increased functional coupling with the PCC. Additionally, as shown from the multiple regression analyses with the ALFF maps, the right OFC and part of the superior parietal lobule (BA 5) showed opposite patterns of correlations with promotion and prevention, respectively. This result may indicate that the achievement motivation orientation of promotion or prevention could be differentiated by the local brain activity within the frontal-parietal interaction.
Several limitations of our study should be considered. First, though the logistic regression model could independently distinguish each of the four motivational orientations, our results demonstrated behavioral and neural differences according to the two original pro/pre and self/other dimensions. To outset this limitation, we performed multiple regression analyses to identify the neural predictors associated with each of the four motivational orientations. Second, it is uncertain whether our neural findings are a causal role for the participants’ behavior or if they resulted from individual behavioral differences, since our sample was not collected for a longitudinal study. Future longitudinal research is thus needed to clarify this issue.
Conclusion
The current study used unsupervised clustering on behavioral responses to categorize people by their similarities in achievement motivation orientations and investigated the associated neural mechanisms using brain imaging data unrelated to the task, including T1-weighted MRI and rsfMRI data. Our results provide novel insights on the morphological features in the PPC underlying the motivational processing of subjective preferences toward promotion- and prevention-focused orientations. This finding was underpinned by multiple regression analysis which verified PPC as a neural predictor for promotion- and prevention-focused motivational orientations. We also confirmed previous findings of functional coupling between the mPFC and PCC within the DMN related to self/other processing. Achievement motivation is a multidimensional process that involves neural networks related to various cognitive functions; our structural and functional findings could allow future identification of individual differences in achievement motivation.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Ames, C. (1992). Classrooms: goals, structures, and student motivation. Journal of Educational Psychology, 84(3), 261–271. https://doi.org/10.1037/0022-0663.84.3.261
Ames, C., & Archer, J. (1988). Achievement goals in the classroom: Students’ learning strategies and motivation processes. Journal of Educational Psychology, 80(3), 260–267. https://doi.org/10.1037/0022-0663.80.3.260
Andrews-Hanna, J. R., Smallwood, J., & Spreng, R. N. (2014). The default network and self-generated thought: Component processes, dynamic control, and clinical relevance. Annals of the New York Academy Sciences, 1316, 29–52. https://doi.org/10.1111/nyas.12360
Antunes, J. A. P. (2021). To supervise or to self-supervise: A machine learning based comparison on credit supervision. Financial Innovation, 7, 26. https://doi.org/10.1186/s40854-021-00242-4
Atkinson, J. W. (1957). Motivational determinants of risk-taking behavior. Psychological Review 64 Part, 1(6), 359–372. https://doi.org/10.1037/h0043445
Badre, D., & Nee, D. E. (2018). Frontal cortex and the hierarchical control of behavior. Trends in Cognitive Sciences, 22(2), 170–188. https://doi.org/10.1016/j.tics.2017.11.005
Bartra, O., McGuire, J. T., & Kable, J. W. (2013). The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage, 76, 412–427. https://doi.org/10.1016/j.neuroimage.2013.02.063
Behzadi, Y., Restom, K., Liau, J., & Liu, T. T. (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage, 37(1), 90–101. https://doi.org/10.1016/j.neuroimage.2007.04.042
Bryan, J. F., & Locke, E. A. (1967). Goal setting as a means of increasing motivation. Journal of Applied Psychology, 51(3), 274–277. https://doi.org/10.1037/h0024566
Buckner, R. L., Andrews-Hanna, J. R., & Schacter, D. L. (2008). The brain’s default network: Anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences, 1124, 1–38. https://doi.org/10.1196/annals.1440.011
Chao-Gan, Y., & Yu-Feng, Z. (2010). DPARSF: A MATLAB toolbox for “Pipeline” data analysis of resting-state fMRI. Frontiers in Systems Neuroscience, 4, 13. https://doi.org/10.3389/fnsys.2010.00013
Corbetta, M., Patel, G., & Shulman, G. L. (2008). The reorienting system of the human brain: From environment to theory of mind. Neuron, 58(3), 306–324. https://doi.org/10.1016/j.neuron.2008.04.017
Dahnke, R., Yotter, R. A., & Gaser, C. (2013). Cortical thickness and central surface estimation. Neuroimage, 65, 336–348. https://doi.org/10.1016/j.neuroimage.2012.09.050
Darnon, C., Dompnier, B., Gilliéron, O., & Butera, F. (2010). The interplay of mastery and performance goals in social comparison: A multiple-goal perspective. Journal of Educational Psychology, 102(1), 212–222. https://doi.org/10.1037/a0018161
Detloff, A. M., Hariri, A. R., & Strauman, T. J. (2020). Neural signatures of promotion versus prevention goal priming: fMRI evidence for distinct cognitive-motivational systems. Personality Neuroscience, 3(e1), 1–15. https://doi.org/10.1017/pen.2019.13
Eddington, K. M., Dolcos, F., Cabeza, R., KR, R. K., & Strauman, T. J. (2007). Neural correlates of promotion and prevention goal activation: An fMRI study using an idiographic approach. Journal of Cognitive Neuroscience, 19(7), 1152–1162. https://doi.org/10.1162/jocn.2007.19.7.1152
Eklund, A., Nichols, T. E., & Knutsson, H. (2016). Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences of the United States of America, 113(28), 7900–7905. https://doi.org/10.1073/pnas.1602413113
Elliot, A. J., & Church, M. A. (1997). A hierarchical model of approach and avoidance achievement motivation. Journal of Personality and Social Psychology, 72(1), 218–232. https://doi.org/10.1037/0022-3514.72.1.218
Elliot, A. J., & McGregor, H. A. (2001). A 2X2 achievement goal framework. Journal of Personality and Social Psychology, 80, 501–519. https://doi.org/10.1037/0022-3514.80.3.501
Elliot, A. J., & Thrash, T. (2010). Approach and avoidance temperament as basic dimensions of personality. Journal of Personality, 78(3), 865–906. https://doi.org/10.1111/j.1467-6494.2010.00636.x
Haber, S. N., & Knutson, B. (2010). The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology : Official Publication Of The American College Of Neuropsychopharmacology, 35(1), 4–26. https://doi.org/10.1038/npp.2009.129
Higgins, E. T. (1997). Beyond pleasure and pain. American Psychologist, 52(12), 1280–1300. https://doi.org/10.1037//0003-066x.52.12.1280
Iyer, A., Lindner, A., Kagan, I., & Andersen, R. A. (2010). Motor preparatory activity in posterior parietal cortex is modulated by subjective absolute value. PLoS Biolology, 8(8), e1000444. https://doi.org/10.1371/journal.pbio.1000444
Jocham, G., Furlong, P. M., Kroger, I. L., Kahn, M. C., Hunt, L. T., & Behrens, T. E. (2014). Dissociable contributions of ventromedial prefrontal and posterior parietal cortex to value-guided choice. Neuroimage, 100, 498–506. https://doi.org/10.1016/j.neuroimage.2014.06.005
Kahnt, T., Park, S. Q., Haynes, J. D., & Tobler, P. N. (2014). Disentangling neural representations of value and salience in the human brain. Proceedings of the National Academy of Sciences of the United States of America, 111(13), 5000–5005. https://doi.org/10.1073/pnas.1320189111
Kim, S. I., Reeve, J., & Bong, M. (2016). Recent developments in neuroscience research on human motivation, Volume 19. Introduction to motivational neuroscience (pp. 1–19). Bingley, UK: Emerald Group Publishing Limited. https://doi.org/10.1108/S0749-742320160000019022
Knutson, B., & Gibbs, S. E. (2007). Linking nucleus accumbens dopamine and blood oxygenation. Psychopharmacology (Berl), 191(3), 813–822. https://doi.org/10.1007/s00213-006-0686-7
Kou, G., Peng, Y., & Wang, G. (2014). Evaluation of clustering algorithms for financial risk analysis using MCDM methods. Information Sciences, 275, 1–12. https://doi.org/10.1016/j.ins.2014.02.137
Kou, G., Yang, P., Peng, Y., Xiao, F., Chen, Y., & Alsaadi, F. E. (2020). Evaluation of feature selection methods for text classification with small datasets using multiple criteria decision-making methods. Applied Soft Computing, 86, 105836. https://doi.org/10.1016/j.asoc.2019.105836
Li, B., & Xu, Z. (2021). Insight into financial technology (FinTech): a bibliometric and visual study. Financial Innovation, 7, 69. https://doi.org/10.1186/s40854-021-00285-7
Maddox, W. T., & Markman, A. B. (2010). The motivation-cognition interface in learning and decision-making. Current Direction in Psychological Science, 19(2), 106–110. https://doi.org/10.1177/0963721410364008
Midgley, C., Maehr, M. L., Hruda, L. Z., Anderman, E., Anderman, L., Freeman, K. E., Gheen, M., Kaplan, A., Kumar, R., Middleton, M. J., Nelson, J., Roeser, R., & Urdan, T. (2000). Manual for the patterns of adaptive learning scales. Ann Arbor, MI: University of Michigan.
Milligan, G. W., & Cooper, M. C. (1985). An examination of procedures for determining the number of clusters in a data set. Psychometrika, 50(2), 159–179. https://doi.org/10.1007/BF02294245
Mojena, R. (1977). Hierarchical grouping methods and stopping rules: An evaluation. The Computer Journal, 20(4), 359–363. https://doi.org/10.1093/comjnl/20.4.359
Murayama, K., Elliot, A. J., & Friedman, R. (2012). Achievement goals. In R. Ryan (Ed.), The Oxford handbook of human motivation (pp. 191–207). Oxford, UK: Oxford University Press. https://doi.org/10.1093/oxfordhb/9780195399820.001.0001
Nicholls, J. G. (1989). The competitive ethos and democratic education. Cambridge, MA: Harvard University Press.
O’Doherty, J. P., Dayan, P., Friston, K., Critchley, H., & Dolan, R. J. (2003). Temporal difference models and reward-related learning in the human brain. Neuron, 38(2), 329–337. https://doi.org/10.1016/s0896-6273(03)00169-7
Park, Y., & Park, S. W. (2017). Goal orientations and social comparison: The role of different motivations in affiliation preferences. Motivation and Emotion, 41(5), 617–627. https://doi.org/10.1007/s11031-017-9634-6
Peck, C. J., Jangraw, D. C., Suzuki, M., Efem, R., & Gottlieb, J. (2009). Reward modulates attention independently of action value in posterior parietal cortex. Journal of Neuroscience, 29(36), 11182–11191. https://doi.org/10.1523/JNEUROSCI.1929-09.2009
Power, J. D., Barnes, K. A., Snyder, A. Z., Schlaggar, B. L., & Petersen, S. E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage, 59(3), 2142–2154. https://doi.org/10.1016/j.neuroimage.2011.10.018
Raichle, M. E., MacLeod, A. M., Snyder, A. Z., Powers, W. J., Gusnard, D. A., & Shulman, G. L. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America, 98(2), 676–682. https://doi.org/10.1073/pnas.98.2.676
Rangel, A., Camerer, C., & Montague, P. R. (2008). A framework for studying the neurobiology of value-based decision making. Nature Reviews Neuroscience, 9(7), 545–556. https://doi.org/10.1038/nrn2357
Régner, I., Escribe, C., & Dupeyrat, C. (2007). Evidence of social comparison in mastery goals in natural academic settings. Journal of Educational Psychology, 99(3), 575–583. https://doi.org/10.1037/0022-0663.99.3.575
Smiley, P. A., & Dweck, C. S. (1994). Individual differences in achievement goals among young children. Child Development, 65, 1723–1743. https://doi.org/10.1111/j.1467-8624.1994.tb00845.x
Strauman, T. J., & Eddington, K. M. (2017). Treatment of depression from a self-regulation perspective: Basic concepts and applied strategies in self-system therapy. Cognitive Therapy and Research, 41(1), 1–15. https://doi.org/10.1007/s10608-016-9801-1
Swanson, S., & Tricomi, E. (2014). Goals and task difficulty expectations modulate striatal responses to feedback. Cognitive Affective & Behavioral Neuroscience, 14(2), 610–620. https://doi.org/10.3758/s13415-014-0269-8
Theeuwes, J. (1994). Endogenous and exogenous control of visual selection. Perception, 23(4), 429–440. https://doi.org/10.1068/p230429
Yang, H., Long, X. Y., Yang, Y., Yan, H., Zhu, C. Z., Zhou, X. P., Zang, Y. F., & Gong, Q. Y. (2007). Amplitude of low frequency fluctuation within visual areas revealed by resting-state functional MRI. Neuroimage, 36(1), 144–152. https://doi.org/10.1016/j.neuroimage.2007.01.054
Zang, Y. F., He, Y., Zhu, C. Z., Cao, Q. J., Sui, M. Q., Liang, M., Tian, L. X., Jiang, T. Z., & Wang, Y. F. (2007). Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain & Development, 29(2), 83–91. https://doi.org/10.1016/j.braindev.2006.07.002
Acknowledgements
The authors would like to thank Dr. Hackjin Kim and Mr. Kun il Kim for their helpful comments on earlier version of the task paradigm at the beginning of this study.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grants funded by the Korea government (MSIT) (Grant Nos. NRF-2019R1G1A1098972 and NRF-2022R1A2C1010704).
Author information
Authors and Affiliations
Contributions
Conceptualization: H. J. Han, W. H. Jung; Methodology: S. Lee; Formal analysis and investigation: H. J. Han, W. H. Jung; Writing – original draft preparation: H. J. Han; Writing - review & editing: H. J. Han, S. Lee, W. H. Jung; Funding acquisition: W. H. Jung; Resources: W. H. Jung; Supervision: W. H. Jung.
Corresponding author
Ethics declarations
Conflict of interest/competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
This study was performed in line with the principles of Declaration of Helsinki. Approval was granted by the Ethics Committee of Daegu University (Feb 21, 2022/NO. 1040621-202201-HR-002).
Informed consent
Written informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Han, H.J., Lee, S. & Jung, W.H. The involvement of the posterior parietal cortex in promotion and prevention focus. Curr Psychol 42, 26115–26124 (2023). https://doi.org/10.1007/s12144-022-03731-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12144-022-03731-6