Abstract
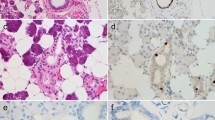
GATA3 is a zinc finger transcription factor that regulates the normal development of many tissues and cell types. Recent studies have shown that immunohistochemical nuclear staining for GATA3 among tumors is highly restricted to carcinomas of breast and urothelial origin; however salivary gland tumors have not been tested. Given that breast and salivary gland tissues are very similar with respect to embryologic development and structure, we performed GATA3 staining on a spectrum of salivary gland neoplasms. GATA3 immunohistochemistry was performed on a diverse collection of 180 benign and malignant salivary gland neoplasms including 10 acinic cell carcinomas, 2 adenocarcinomas not otherwise specified, 41 adenoid cystic carcinomas, 2 epithelial-myoepithelial carcinomas, 1 low grade cribriform cystadenocarcinoma, 15 mammary analogue secretory carcinomas, 7 metastatic squamous cell carcinomas, 27 mucoepidermoid carcinomas, 2 oncocytic carcinomas, 5 oncocytomas, 34 pleomorphic adenomas, 4 polymorphous low grade adenocarcinomas, 25 salivary duct carcinomas, and 5 Warthin tumors. Staining for GATA3 was observed in 92/180 (51 %) of salivary gland tumors. GATA3 staining was observed in most of the tumor types, but diffuse immunolabeling was consistently seen in salivary duct carcinoma (25 of 25) and mammary analogue secretory carcinoma (15 of 15)—the two tumor types that most closely resemble breast neoplasia. Background benign salivary gland tissue was also usually weakly positive in both acini and ducts. GATA3 immunostaining is not restricted to tumors of breast and urothelial origin. Rather, it is expressed across many different types of salivary gland neoplasms. As a result, salivary gland origin should be considered in the differential diagnosis of a GATA3-positive carcinoma, particularly in the head and neck. Although GATA3 immunohistochemistry is not helpful in resolving the differential diagnosis between a primary salivary gland neoplasm and metastatic breast cancer, it may have some utility in subtyping salivary gland tumors, particularly salivary duct carcinoma and mammary analogue secretory carcinoma.


Similar content being viewed by others
References
Kouros-Mehr H, Slorach EM, Sternlicht MD, et al. GATA-3 maintains the differentiation of the luminal cell fate in the mammary gland. Cell. 2006;127:1041–55.
Asselin-Labat ML, Sutherland KD, Barker H, et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol. 2007;9:201–9.
Hendriks RW, Nawijn MC, Engel JD, et al. Expression of the transcription factor GATA-3 is required for the development of the earliest T cell progenitors and correlates with stages of cellular proliferation in the thymus. Eur J Immunol. 1999;29:1912–8.
Grote D, Souabni A, Busslinger M, et al. Pax 2/8-regulated Gata 3 expression is necessary for morphogenesis and guidance of the nephric duct in the developing kidney. Development. 2006;133:53–61.
Labastie MC, Catala M, Gregoire JM, et al. The GATA-3 gene is expressed during human kidney embryogenesis. Kidney Int. 1995;47:1597–603.
Tsarovina K, Pattyn A, Stubbusch J, et al. Essential role of Gata transcription factors in sympathetic neuron development. Development. 2004;131:4775–86.
Kaufman CK, Zhou P, Pasolli HA, et al. GATA-3: an unexpected regulator of cell lineage determination in skin. Genes Dev. 2003;17:2108–22.
Liu H, Shi J, Wilkerson ML, et al. Immunohistochemical evaluation of GATA3 expression in tumors and normal tissues: a useful immunomarker for breast and urothelial carcinomas. Am J Clin Pathol. 2012;138:57–64.
Higgins JP, Kaygusuz G, Wang L, et al. Placental S100 (S100P) and GATA3: markers for transitional epithelium and urothelial carcinoma discovered by complementary DNA microarray. Am J Surg Pathol. 2007;31:673–80.
Yang M, Nonaka D. A study of immunohistochemical differential expression in pulmonary and mammary carcinomas. Mod Pathol. 2010;23:654–61.
Chang A, Amin A, Gabrielson E, et al. Utility of GATA3 immunohistochemistry in differentiating urothelial carcinoma from prostate adenocarcinoma and squamous cell carcinomas of the uterine cervix, anus, and lung. Am J Surg Pathol. 2012;36:1472–6.
Gruver AM, Amin MB, Luthringer DJ, et al. Selective immunohistochemical markers to distinguish between metastatic high-grade urothelial carcinoma and primary poorly differentiated invasive squamous cell carcinoma of the lung. Arch Pathol Lab Med. 2012;136:1339–46.
Skalova A, Vanecek T, Sima R, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol. 2010;34:599–608.
Bishop JA, et al. Cytopathologic features of mammary analogue secretory carcinoma. Cancer Cytopathol. 2012. doi:10.1002/cncy.21245.
Bishop JA, Yonescu R, Batista D, Eisele DW, et al. Most non-parotid “acinic cell carcinomas” represent mammary analoge secretory carcinomas. Am J Surg Pathol. 2013;In Press.
Engelsen IB, Stefansson IM, Akslen LA, et al. GATA3 expression in estrogen receptor alpha-negative endometrial carcinomas identifies aggressive tumors with high proliferation and poor patient survival. Am J Obstet Gynecol. 2008; 199: 543 e541–547.
Wick MR, Ockner DM, Mills SE, et al. Homologous carcinomas of the breasts, skin, and salivary glands. A histologic and immunohistochemical comparison of ductal mammary carcinoma, ductal sweat gland carcinoma, and salivary duct carcinoma. Am J Clin Pathol. 1998;109:75–84.
Swanson PE, Pettinato G, Lillemoe TJ, et al. Gross cystic disease fluid protein-15 in salivary gland tumors. Arch Pathol Lab Med. 1991;115:158–63.
Wick MR, Lillemoe TJ, Copland GT, Swanson PE, Manivel JC, Kiang DT. Gross cystic disease fluid protein-15 as a marker for breast cancer: immunohistochemical analysis of 690 human neoplasms and comparison with alpha-lactalbumin. Hum Pathol. 1989;20:281–7.
Ellis GL, Auclair PL. Salivary duct carcinoma. AFIP atlas of tumor pathology: tumors of the salivary glands. Washington, D.C.: ARP Press, 2008:322–332.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schwartz, L.E., Begum, S., Westra, W.H. et al. GATA3 Immunohistochemical Expression in Salivary Gland Neoplasms. Head and Neck Pathol 7, 311–315 (2013). https://doi.org/10.1007/s12105-013-0442-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-013-0442-3