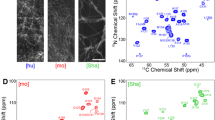
Abstract
Polymorphism is a common and important phenomenon for protein fibrils which has been linked to the appearance of strains in prion and other neurodegenerative diseases. Parkinson disease is a frequently occurring neurodegenerative pathology, tightly associated with the formation of Lewy bodies. These deposits mainly consist of α-synuclein in fibrillar, β-sheet-rich form. α-synuclein is known to form numerous different polymorphs, which show distinct structural features. Here, we describe the chemical shift assignments, and derive the secondary structure, of a polymorph that was fibrillized at higher-than-physiological pH conditions. The fibrillar core contains residues 40–95, with both the C- and N-terminus not showing any ordered, rigid parts. The chemical shifts are similar to those recorded previously for an assigned polymorph that was fibrillized at neutral pH.





Similar content being viewed by others
References
Böckmann A, Gardiennet C, Verel R et al (2009) Characterization of different water pools in solid-state NMR protein samples. J Biomol NMR 45:319–327. doi:10.1007/s10858-009-9374-3
Bousset L, Pieri L, Ruiz-Arlandis G et al (2013) Structural and functional characterization of two alpha-synuclein strains. Nat Commun 4:2575. doi:10.1038/ncomms3575
Brundin P, Melki R, Kopito R (2010) PeRsPecTives. 1–7. doi: 10.1038/nrm2873
Cohlberg JA, Li J, Uversky VN, Fink AL (2002) Heparin and other glycosaminoglycans stimulate the formation of amyloid fibrils from α-synuclein in vitro. Biochemistry 41:1502–1511. doi:10.1021/bi011711s
Comellas G, Lemkau LR, Nieuwkoop AJ et al (2011) Structured regions of α-synuclein fibrils include the early-onset Parkinson’s disease mutation sites. J Mol Biol 411:881–895. doi:10.1016/j.jmb.2011.06.026
Comellas G, Lemkau LR, Zhou DH et al (2012) Structural intermediates during α-synuclein fibrillogenesis on phospholipid vesicles. J Am Chem Soc 134:5090–5099. doi:10.1021/ja209019s
Cornilescu G, Delaglio F, Bax A (1999) Protein backbone angle restraints from searching a database for chemical shift and sequence homology. J Biomol NMR 13:289–302. doi:10.1023/A:1008392405740
Der-Sarkissian A, Jao CC, Chen J et al (2003) Structural organization of alpha-synuclein fibrils studied by site-directed spin labeling. J Biol Chem 278:37530–37535. doi:10.1074/jbc.M305266200
Eller M, William DR (2011) α-Synuclein in Parkinson disease and other neurodegenerative disorders. Clin Chem Lab Med 49:403–408. doi:10.1515/CCLM.2011.077
Gath J, Habenstein B, Bousset L et al (2012) Solid-state NMR sequential assignments of α-synuclein. Biomol NMR Assign 6:51–55. doi:10.1007/s12104-011-9324-3
Gath J, Bousset L, Habenstein B et al (2014a) Unlike twins: an NMR comparison of two α-synuclein polymorphs featuring different toxicity. PLoS ONE 9:e90659. doi:10.1371/journal.pone.0090659
Gath J, Bousset L, Habenstein B et al (2014b) Yet another polymorph of α-synuclein: solid-state sequential assignments. Biomol NMR Assign 8:395–404. doi:10.1007/s12104-013-9526-y
Habenstein B, Wasmer C, Bousset L et al (2011) Extensive de novo solid-state NMR assignments of the 33 kDa C-terminal domain of the Ure2 prion. J Biomol NMR 51:235–243. doi:10.1007/s10858-011-9530-4
Hansen C, Angot E, Bergström AL et al (2011) α-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest 121:715–725. doi:10.1172/JCI43366
Heise H, Hoyer W, Becker S et al (2005) Molecular-level secondary structure, polymorphism, and dynamics of full-length alpha-synuclein fibrils studied by solid-state NMR. Proc Natl Acad Sci USA 102:15871–15876. doi:10.1073/pnas.0506109102
Lv G, Kumar A, Giller K et al (2012) Structural comparison of mouse and human α-synuclein amyloid fibrils by solid-state NMR. J Mol Biol 420:99–111. doi:10.1016/j.jmb.2012.04.009
Miake H (2002) Biochemical characterization of the core structure of alpha-synuclein filaments. J Biol Chem 277:19213–19219. doi:10.1074/jbc.M110551200
Pawar AP, DuBay KF, Zurdo J, Chiti F, Vendruscolo M, Dobson CM (2005) Prediction of “aggregation-prone” and “aggregation-susceptible” regions in proteins associated with neurodegenerative diseases. J Mol Biol 350:379–392. doi:10.1016/j.jmb.2005.04.016
Schuetz A, Wasmer C, Habenstein B et al (2010) Protocols for the sequential solid-state NMR spectroscopic assignment of a uniformly labeled 25 kDa protein: HET-s(1-227). ChemBioChem 11:1543–1551. doi:10.1002/cbic.201000124
Stevens TJ, Fogh RH, Boucher W et al (2011) A software framework for analysing solid-state MAS NMR data. J Biomol NMR 51:437–447. doi:10.1007/s10858-011-9569-2
Acknowledgments
This work was supported by the Agence Nationale de la Recherche (ANR-11-BSV8-021-01, ANR-12-BS08-0013-01), the ETH Zurich, the Swiss National Science Foundation (Grant 200020_124611), the Era-Net Neuron (Project MIPROTRAN, ANR-08-NEUR-001-01) and the Centre National de la Recherche Scientifique. We also acknowledge support from the European Commission under the Seventh Framework Programme (FP7), contract Bio-NMR 261863.
Author information
Authors and Affiliations
Corresponding author
Additional information
Joeri Verasdonck and Luc Bousset have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Verasdonck, J., Bousset, L., Gath, J. et al. Further exploration of the conformational space of α-synuclein fibrils: solid-state NMR assignment of a high-pH polymorph. Biomol NMR Assign 10, 5–12 (2016). https://doi.org/10.1007/s12104-015-9628-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-015-9628-9