Abstract
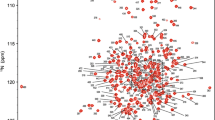
Lon is an ATPases associated with diverse cellular activities protease and belongs to a unique group that binds DNA. The α sub-domain of Lon protease is responsible for DNA-binding, but the structural information for its DNA-recognition mode is still limited. Here, we report 1H, 15N and 13C backbone assignment for the α sub-domain from Brevibacillus thermoruber Lon protease as the basis for the elucidation of its structure and interactions with DNA, necessary for understanding the allosteric regulatory mechanism of the enzymatic function.


Similar content being viewed by others
References
Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6(3):277–293
Fukui T, Eguchi T, Atomi H, Imanaka T (2002) A membrane-bound archaeal Lon protease displays ATP-independent proteolytic activity towards unfolded proteins and ATP-dependent activity for folded proteins. J Bacteriol 184(13):3689–3698
Goldberg AL, Moerschell RP, Chung CH, Maurizi MR (1994) ATP-dependent protease La (lon) from Escherichia coli. Methods Enzymol 244:350–375
Gottesman S (1996) Proteases and their targets in Escherichia coli. Annu Rev Genet 30:465–506
Johnson BA, Blevins RA (1994) NMRView: a computer program for the visualization and analysis of NMR data. J Biomol NMR 4(5):603–614
Lee I, Suzuki CK (2008) Functional mechanics of the ATP-dependent Lon protease- lessons from endogenous protein and synthetic peptide substrates. Biochim Biophys Acta 1784(5):727–735
Lee AY, Hsu CH, Wu SH (2004a) Functional domains of Brevibacillus thermoruber lon protease for oligomerization and DNA binding: role of N-terminal and sensor and substrate discrimination domains. J Biol Chem 279(33):34903–34912
Lee AY, Tsay SS, Chen MY, Wu SH (2004b) Identification of a gene encoding Lon protease from Brevibacillus thermoruber WR-249 and biochemical characterization of its thermostable recombinant enzyme. Eur J Biochem 271(4):834–844
Lin YC, Lee HC, Wang I, Hsu CH, Liao JH, Lee AY, Chen C, Wu SH (2009) DNA-binding specificity of the Lon protease alpha-domain from Brevibacillus thermoruber WR-249. Biochem Biophys Res Commun 388(1):62–66
Markley JL, Bax A, Arata Y, Hilbers CW, Kaptein R, Sykes BD, Wright PE, Wuthrich K (1998) Recommendations for the presentation of NMR structures of proteins and nucleic acids. IUPAC–IUBMB–IUPAB Inter-Union Task Group on the standardization of data bases of protein and nucleic acid structures determined by NMR spectroscopy. J Biomol NMR 12(1):1–23
Neuwald AF, Aravind L, Spouge JL, Koonin EV (1999) AAA+: a class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res 9(1):27–43
Rudyak SG, Brenowitz M, Shrader TE (2001) Mg2+-linked oligomerization modulates the catalytic activity of the Lon (La) protease from Mycobacterium smegmatis. Biochemistry 40(31):9317–9323
Schmidt R, Decatur AL, Rather PN, Moran CP Jr, Losick R (1994) Bacillus subtilis lon protease prevents inappropriate transcription of genes under the control of the sporulation transcription factor sigma G. J Bacteriol 176(21):6528–6537
Shen Y, Delaglio F, Cornilescu G, Bax A (2009) TALOS+: a hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J Biomol NMR 44(4):213–223
Smith CK, Baker TA, Sauer RT (1999) Lon and Clp family proteases and chaperones share homologous substrate-recognition domains. Proc Natl Acad Sci USA 96(12):6678–6682
Stahlberg H, Kutejova E, Suda K, Wolpensinger B, Lustig A, Schatz G, Engel A, Suzuki CK (1999) Mitochondrial Lon of Saccharomyces cerevisiae is a ring-shaped protease with seven flexible subunits. Proc Natl Acad Sci USA 96(12):6787–6790
van Dyck L, Pearce DA, Sherman F (1994) PIM1 encodes a mitochondrial ATP-dependent protease that is required for mitochondrial function in the yeast Saccharomyces cerevisiae. J Biol Chem 269(1):238–242
Vasilyeva OV, Kolygo KB, Leonova YF, Potapenko NA, Ovchinnikova TV (2002) Domain structure and ATP-induced conformational changes in Escherichia coli protease Lon revealed by limited proteolysis and autolysis. FEBS Lett 526(1–3):66–70
Wang N, Gottesman S, Willingham MC, Gottesman MM, Maurizi MR (1993) A human mitochondrial ATP-dependent protease that is highly homologous to bacterial Lon protease. Proc Natl Acad Sci USA 90(23):11247–11251
Zehnbauer BA, Foley EC, Henderson GW, Markovitz A (1981) Identification and purification of the Lon+ (capR+) gene product, a DNA-binding protein. Proc Natl Acad Sci USA 78(4):2043–2047
Acknowledgments
The NMR spectra were obtained at NMR facility of instrumentation center in NTU and High-Field Nuclear Magnetic Resonance Center (HF-NMRC) supported by the National Research Program for Genomic Medicine. This work was supported by the National Science Council (NSC99-2119-M-002-010) and National Taiwan University (NTU-ERP-101R8600-1 and NTU-ICRP-102R7560-5) to C.-H. Hsu.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, YD., Wu, SH. & Hsu, CH. Backbone resonance assignments of the α sub-domain of Brevibacillus thermoruber Lon protease. Biomol NMR Assign 8, 233–236 (2014). https://doi.org/10.1007/s12104-013-9490-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-013-9490-6