Abstract
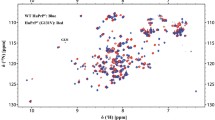
The sequence specific backbone 1H, 13C and 15N resonance assignments of an intrinsically unstructured βγ-crystallin from Hahella chejuensis are reported. The secondary structure chracterization of the unstructured protein reveals that large fraction of residues exhibits β-strand propensity, as in the case of the Ca2+-bound structured protein.


Similar content being viewed by others
References
Aravind P, Suman SK, Mishra A, Sharma Y, Sankaranarayanan R (2009) βγ-crystallin superfamily contains a universal motif for binding calcium. Biochemistry 48:12180–12190
Atreya HS, Sahu SC, Chary KVR, Govil G (2000) A tracked approach for automated NMR assignments in proteins (TATAPRO). J Biomol NMR 17:125–136
Atreya HS, Chary KVR, Govil G (2002) Automated NMR assignments of proteins for high throughput structure determination: TATAPRO II. Curr Sci 83:1372–1375
Bax A, Grzesiek S (1993) Methodological advances in protein NMR. Acc Chem Res 26:131–138
Bax A, Ikura M, Kay LE, Barbato G, Spera S (1991) Multidimensional triple resonance NMR spectroscopy of isotopically uniformly enriched proteins: a powerful new strategy for structure determination. Ciba Found Symp 161:108–119
Edison AS, Abildgaard F, Westler WM, Mooberry ES, Markley JL (1994) Practical introduction to theory and implementation of multinuclear, multidimensional nuclear magnetic resonance experiments. Methods Enzymol 239:3–79
Gao Q, Xiang Y, Zeng L, Ma XT, Lee WH, Zhang Y (2011) Characterization of the βγ-crystallin domains of βγ-CAT, a non-lens βγ-crystallin and trefoil factor complex, from the skin of the toad Bombina maxima. Biochimie 93:1865–1872
Graw J (2009) Genetics of crystallins: cataract and beyond. Exp Eye Res 88:173–189
Jobby MK, Sharma Y (2005) Calcium-binding crystallins from Yersinia pestis. J Biol Chem 280:1209–1216
Jobby MK, Sharma Y (2007) Caulollins from Caulobacter crescentus, a pair of partially unstructured proteins of βγ-crystallin superfamily, gain structure upon binding calcium. Biochemistry 46:12298–12307
Keller RLJ (2004) The computer aided NMR resonance assignment tutorial. CANTINA Verlag, Switzerland
Kretschmar M, Mayr E-M, Jaenicke R (1999) Kinetic and thermodynamic stabilization of the βγ-crystallin homolog spherulin 3a from Physarum polycephalum by calcium binding. J Mol Biol 289:701–705
Ma B, Sen T, Asnaghi L, Valapala M, Yang F, Hose S, McLeod DS, Lu Y, Eberhart C, Zigler JS Jr, Sinha D (2011) βA3/A1-crystallin controls anoikis-mediated cell death in astrocytes by modulating PI3K/AKT/mTOR and ERK survival pathways through the PKD/Bit1-signaling axis. Cell Death Dis. doi:10.1038/cddis.2011.100
Metzler WJ, Constantine KL, Friedrichs MS, Bell AJ, Ernst EG, Lavoie TB, Mueller L (1993) Characterization of the three-dimensional solution structure of human profilin: 1H, 13C, and 15N NMR assignments and global folding pattern. Biochemistry 32:13818–13829
Mishra A, Suman SK, Srivastava SS, Sankaranarayanan R, Sharma Y (2012) Decoding the molecular design principles underlying Ca2+-binding to βγ-crystallin motifs. J Mol Biol 415:75–91
Srivastava AK, Chary KVR (2011) Conformational heterogeneity and dynamics in a βγ-crystallin from Hahella chejuensis. Biophys Chem 157:7–15
Srivastava AK, Sharma Y, Chary KVR (2008) Overexpression, on-column refolding and isotopic labeling of Hahellin from Hahella chejuensis, a putative member of the βγ-crystallin superfamily. Protein Expr and Purif 58:269–274
Srivastava AK, Sharma Y, Chary KVR (2010) A natively unfolded βγ-crystallin domain from Hahella chejuensis. Biochemistry 49:9746–9755
Suman SK, Mishra A, Ravindra D, Yeramala L, Sharma Y (2011) Evolutionary remodelling of the betagamma-crystallins for domain stability at the cost of Ca2+-binding. J Biol Chem 286:43891–43901
Wenk M, Mayr E-M (1998) Myxococcus xanthus spore coat protein S, a stress-induced member of the βγ-crystallin superfamily, gains stability from binding of calcium ions. Eur J Biochem 255:604–610
Wistow G (1990) Evolution of a protein superfamily: relationships between vertebrate lens crystallins and microorganism dormancy proteins. J Mol Evol 30:140–145
Zhang H, Neal S, Wishart DS (2003) A database of uniformly referenced protein chemical shifts. J Biomol NMR 25:173–195
Acknowledgement
The facilities provided by the National Facility for High Field NMR, supported by the Department of Science and Technology (DST), Department of Biotechnology (DBT), Council of Scientific and Industrial Research (CSIR), and Tata Institute of Fundamental Research (TIFR), Mumbai, are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramanujam, V., Patel, S., Srivastava, A.K. et al. Backbone 1H, 13C and 15N resonance assignments of an intrinsically unstructured βγ-crystallin from Hahella chejuensis . Biomol NMR Assign 7, 221–224 (2013). https://doi.org/10.1007/s12104-012-9414-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-012-9414-x